Long-Term Outcomes after Use of Perioperative Glucocorticoids in Patients Undergoing Cancer Surgery: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Protocol and Registration
2.2. Eligibility Criteria
2.3. Search Strategy for Identification of Studies
2.4. Study Selection and Data Collection
2.5. Risk of Bias in Individual Studies
2.6. Statistical Analyses
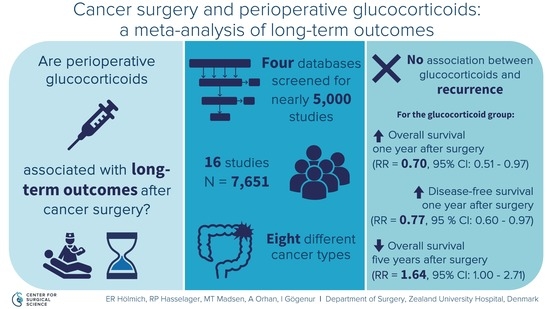
3. Results
3.1. Study Selection
3.2. Study and Patient Characteristics
3.3. Risk of Bias within Studies
3.4. Recurrence
3.5. Secondary Outcomes
Overall Survival
3.6. Disease-Free Survival
3.7. Cancer Specific Survival
3.8. Subgroup Analyses
3.9. Sensitivity Analysis
3.10. Risk of Bias across Studies
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chaffer, C.L.; Weinberg, R.A. A perspective on cancer cell metastasis. Science 2011, 331, 1559–1564. [Google Scholar] [CrossRef] [PubMed]
- International Agency for Research on Cancer, C.T. Available online: http://gco.iarc.fr/tomorrow/graphic-isotype?type=0&population=900&mode=population&sex=0&cancer=39&age_group=value&apc_male=0&apc_female=0 (accessed on 16 October 2019).
- Freeman, J.; Connolly, C.; Buggy, D. Mechanisms of metastasis of solid organ tumors in the perioperative period. Int. Anesthesiol. Clin. 2016, 54, 29–47. [Google Scholar] [CrossRef]
- Horowitz, M.; Neeman, E.; Sharon, E.; Ben-Eliyahu, S. Exploiting the critical perioperative period to improve long-term cancer outcomes. Nat. Rev. Clin. Oncol. 2015, 12, 213–226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ricon, I.; Hanalis-Miller, T.; Haldar, R.; Jacoby, R.; Ben-Eliyahu, S. Perioperative biobehavioral interventions to prevent cancer recurrence through combined inhibition of beta-adrenergic and cyclooxygenase 2 signaling. Cancer 2019, 125, 45–56. [Google Scholar] [CrossRef] [Green Version]
- Momosaki, K.; Ishibashi, N.; Yoshida, S.; Muraoka, T.; Tanaka, K.; Iwakuma, N.; Oka, Y.; Kaibara, A.; Akagi, Y.; Shirouzu, K. Effect of preoperative administration of methylpredonisolone and ulinastatin on tumor cell metastasis after surgical stress. Kurume Med. J. 2014, 60, 79–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Egberts, J.H.; Schniewind, B.B.; Patzold, M.; Kettler, B.; Tepel, J.; Kalthoff, H.; Trauzold, A. Dexamethasone reduces tumor recurrence and metastasis after pancreatic tumor resection in SCID mice. Cancer Biol. Ther. 2008, 7, 1044–1050. [Google Scholar] [CrossRef] [Green Version]
- Rosenne, E.; Sorski, L.; Shaashua, L.; Neeman, E.; Matzner, P.; Levi, B.; Ben-Eliyahu, S. In vivo suppression of NK cell cytotoxicity by stress and surgery: Glucocorticoids have a minor role compared to catecholamines and prostaglandins. Brain Behav. Immun. 2014, 37, 207–219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Srinivasa, S.; Kahokehr, A.A.; Yu, T.C.; Hill, A.G. Preoperative glucocorticoid use in major abdominal surgery: Systematic review and meta-analysis of randomized trials. Ann. Surg. 2011, 254, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Sauerland, S.; Nagelschmidt, M.; Mallmann, P.; Neugebauer, E.A. Risks and benefits of preoperative high dose methylprednisolone in surgical patients: A systematic review. Drug Saf. 2000, 23, 449–461. [Google Scholar] [CrossRef] [PubMed]
- Buggy, D.J.; Borgeat, A.; Cata, J.; Doherty, D.G.; Doornebal, C.W.; Forget, P.; Gottumukkala, V.; Gottschalk, A.; Gupta, A.; Gupta, K.; et al. Consensus statement from the BJA Workshop on Cancer and Anaesthesia. Br. J. Anaesth. 2015, 114, 2–3. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; Group, P.-P. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coffey, J.C.; Wang, J.H.; Smith, M.J.; Bouchier-Hayes, D.; Cotter, T.G.; Redmond, H.P. Excisional surgery for cancer cure: Therapy at a cost. Lancet. Oncol. 2003, 4, 760–768. [Google Scholar] [CrossRef]
- Tierney, J.F.; Stewart, L.A.; Ghersi, D.; Burdett, S.; Sydes, M.R. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007, 8, 16. [Google Scholar] [CrossRef] [Green Version]
- Higgins, J.P.T.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savović, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wells, G.A.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle–Ottawa Scale (NOS) for Assessing the Quality of Non-Randomized Studies in Meta-Analysis. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 23 July 2019).
- Cochrane Collaboration. Review Manager (RevMan) [Computer Program]; Version 5.3; The Nordic Cochrane Centre, The Cochrane Collaboration: Copenhagen, Denmark, 2014. [Google Scholar]
- Call, T.R.; Pace, N.L.; Thorup, D.B.; Maxfield, D.; Chortkoff, B.; Christensen, J.; Mulvihill, S.J. Factors associated with improved survival after resection of pancreatic adenocarcinoma: A multivariable model. Anesthesiology 2015, 122, 317–324. [Google Scholar] [CrossRef]
- Cata, J.P.; Jones, J.; Sepesi, B.; Mehran, R.J.; Rodriguez-Restrepo, A.; Lasala, J.; Feng, L.; Gottumukkala, V. Lack of association between dexamethasone and long-term survival after non-small cell lung cancer surgery. J. Cardiothorac. Vasc. Anesth. 2016, 30, 930–935. [Google Scholar] [CrossRef]
- De Oliveira, G.S., Jr.; McCarthy, R.; Turan, A.; Schink, J.C.; Fitzgerald, P.C.; Sessler, D.I. Is dexamethasone associated with recurrence of ovarian cancer? Anesth. Analg. 2014, 118, 1213–1218. [Google Scholar] [CrossRef]
- Gan, Z.M.; Wang, X.D.; Lv, D.H.; Liu, D.; Li, L. Perioperative immunomodulatory therapy does not decrease postoperative recurrence rate of rectal cancer. Nan Fang Yi Ke Da Xue Xue Bao 2015, 35, 562–566. [Google Scholar] [CrossRef]
- Huang, W.W.; Zhu, W.Z.; Mu, D.L.; Ji, X.Q.; Nie, X.L.; Li, X.Y.; Wang, D.X.; Ma, D. Perioperative management may improve long-term survival in patients after lung cancer surgery: A retrospective cohort study. Anesth. Analg. 2018, 126, 1666–1674. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.H.; Kim, D.W.; Park, S.; Kim, J.H.; Lee, K.Y.; Hwang, J.; Yoo, Y.C. Single dose of dexamethasone is not associated with postoperative recurrence and mortality in breast cancer patients: A propensity-matched cohort study. BMC Cancer 2019, 19, 251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lazzara, C.; Cristina, D.; Iman, K.; Saverio, L.; Angela, A.; Giuseppe, N.; Giuseppe, C. Impact of preoperative corticosteroids on oncological outcomes following colorectal surgery for cancer. Immunol. Endocr. Metab. Agents Med. Chem. 2018, 18, 68–75. [Google Scholar] [CrossRef]
- Merk, B.A.; Havrilesky, L.J.; Ehrisman, J.A.; Broadwater, G.; Habib, A.S. Impact of postoperative nausea and vomiting prophylaxis with dexamethasone on the risk of recurrence of endometrial cancer. Curr. Med. Res. Opin. 2016, 32, 453–458. [Google Scholar] [CrossRef] [PubMed]
- Okazumi, S.; Ochiai, T.; Shimada, H.; Matsubara, H.; Nabeya, Y.; Miyazawa, Y.; Shiratori, T.; Aoki, T.; Sugaya, M. Development of less invasive surgical procedures for thoracic esophageal cancer. Dis. Esophagus 2004, 17, 159–163. [Google Scholar] [CrossRef] [PubMed]
- Sandini, M.; Ruscic, K.J.; Ferrone, C.R.; Warshaw, A.L.; Qadan, M.; Eikermann, M.; Lillemoe, K.D.; Fernandez-Del Castillo, C. Intraoperative dexamethasone decreases infectious complications after pancreaticoduodenectomy and is associated with long-term survival in pancreatic cancer. Ann. Surg. Oncol. 2018, 25, 4020–4026. [Google Scholar] [CrossRef] [PubMed]
- Sato, N.; Koeda, K.; Ikeda, K.; Kimura, Y.; Aoki, K.; Iwaya, T.; Akiyama, Y.; Ishida, K.; Saito, K.; Endo, S. Randomized study of the benefits of preoperative corticosteroid administration on the postoperative morbidity and cytokine response in patients undergoing surgery for esophageal cancer. Ann. Surg. 2002, 236, 184–190. [Google Scholar] [CrossRef]
- Shimada, H.; Okazumi, S.; Matsubara, H.; Nabeya, Y.; Hayashi, H.; Shiratori, T.; Aoki, T.; Shuto, K.; Gunji, Y.; Ochiai, T. Effect of steroid therapy on postoperative course and survival of patients with thoracic esophageal carcinoma. Esophagus 2004, 1, 89–94. [Google Scholar] [CrossRef]
- Singh, P.P.; Lemanu, D.P.; Taylor, M.H.; Hill, A.G. Association between preoperative glucocorticoids and long-term survival and cancer recurrence after colectomy: Follow-up analysis of a previous randomized controlled trial. Br. J. Anaesth. 2014, 113 (Suppl. 1), i68–i73. [Google Scholar] [CrossRef] [Green Version]
- Yano, M.; Taniguchi, M.; Tsujinaka, T.; Fujiwara, Y.; Yasuda, T.; Shiozaki, H.; Monden, M. Is preoperative methylprednisolone beneficial for patients undergoing esophagectomy? Hepato Gastroenterol. 2005, 52, 481–485. [Google Scholar]
- Yu, H.C.; Luo, Y.X.; Peng, H.; Kang, L.; Huang, M.J.; Wang, J.P. Avoiding perioperative dexamethasone may improve the outcome of patients with rectal cancer. Eur. J. Surg. Oncol. 2015, 41, 667–673. [Google Scholar] [CrossRef]
- Zhu, W.Z.; Ji, X.Q.; Tan, H.Y. Retrospective cohort study of the association between perioperative factors and long-term survival after lung cancer operation. Chin. J. Cancer Prev. Treat. 2017, 24, 1387–1391. [Google Scholar] [CrossRef]
- IntHout, J.; Ioannidis, J.P.A.; Borm, G.F. The Hartung-Knapp-Sidik-Jonkman method for random effects meta-analysis is straightforward and considerably outperforms the standard DerSimonian-Laird method. BMC Med. Res. Methodol. 2014, 14, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- IntHout, J.; Ioannidis, J.P.A.; Rovers, M.M.; Goeman, J.J. Plea for routinely presenting prediction intervals in meta-analysis. BMJ Open 2016, 6, e010247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Higgins, J.P.T.; Thompson, S.G.; Spiegelhalter, D.J. A re-evaluation of random-effects meta-analysis. J. R. Stat. Soc. Ser. A Stat. Soc. 2009, 172, 137–159. [Google Scholar] [CrossRef] [Green Version]
- Egger, M.; Smith, G.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.X. Effect of Perioperative Epidural Block and Dexamethasone on Outcome of Patients Undergoing Pancreatic Cancer Surgery; Peking University First Hospital: Beijing, China, 2019. [Google Scholar]
- Zargar-Shoshtari, K.; Sammour, T.; Kahokehr, A.; Connolly, A.B.; Hill, A.G. Randomized clinical trial of the effect of glucocorticoids on peritoneal inflammation and postoperative recovery after colectomy. Br. J. Surg. 2009, 96, 1253–1261. [Google Scholar] [CrossRef]
- McSorley, S.T.; Roxburgh, C.S.D.; Horgan, P.G.; McMillan, D.C. The impact of preoperative dexamethasone on the magnitude of the postoperative systemic inflammatory response and complications following surgery for colorectal cancer. Ann. Surg. Oncol. 2017, 24, 2104–2112. [Google Scholar] [CrossRef] [Green Version]
- Stahn, C.; Buttgereit, F. Genomic and nongenomic effects of glucocorticoids. Nat. Clin. Pract. Rheumatol. 2008, 4, 525–533. [Google Scholar] [CrossRef]
- Gan, T.J.; Diemunsch, P.; Habib, A.S.; Kovac, A.; Kranke, P.; Meyer, T.A.; Watcha, M.; Chung, F.; Angus, S.; Apfel, C.C.; et al. Consensus guidelines for the management of postoperative nausea and vomiting. Anesth. Analg. 2014, 118, 85–113. [Google Scholar] [CrossRef] [Green Version]
- Byrne, K.; Levins, K.; Buggy, D. Can anesthetic-analgesic technique during primary cancer surgery affect recurrence or metastasis? Can. J. Anaesth. 2016, 63, 184–192. [Google Scholar] [CrossRef]
- Amato, A.; Pescatori, M. Perioperative blood transfusions for the recurrence of colorectal cancer. Cochrane Database Syst. Rev. 2006. [Google Scholar] [CrossRef] [PubMed]
- DREAMS Trial Collaborators and West Midlands Research Collaborative. Dexamethasone versus standard treatment for postoperative nausea and vomiting in gastrointestinal surgery: Randomised controlled trial (DREAMS Trial). BMJ 2017, 357, j1455. [Google Scholar] [CrossRef] [Green Version]








| Study (Publication Year) | Study Type | Trial Recruitment Period | Inclusion Criteria (n) | Exclusion Criteria (n) | Exposure/Intervention (n) | Control Group (n) | Reported Outcome(s) |
|---|---|---|---|---|---|---|---|
| Call et al (2015) [19] | Retrospective cohort study | 2001–2012 | -Surgical resection of pancreatic adenocarcinoma stage I–IV | -Insufficient or illegible patient records (26) | Intraoperative dexamethasone treatment (1–10 mg) (69) | No intraoperative dexamethasone treatment (75) | OS |
| Cata et al (2016) [20] | Retrospective cohort study | 2004–2014 | -Surgery with curative intent of NSCLC stage I–III | -Palliative surgery -Secondary malignancy | Intraoperative dexamethasone treatment (4–20 mg) (439) | No intraoperative IV dexamethasone treatment (1110) | OS, DFS |
| De Oliveira et al (2014) [21] | Retrospective cohort study | 1997–2007 | -Optimal cytoreductive surgery of primary ovarian cancer stage I–IV | -Peritoneal tumour, benign or inconclusive pathology (45) -Tumour histology other than ovarian cancer (24) -Secondary surgical procedures (12) -Sub optimally debulked (5) | Intraoperative IV dexamethasone treatment (4–10 mg) (102) | No intraoperative IV dexamethasone treatment (158) | Recurrence |
| Gan et al (2015) [22] | Double-blinded randomized controlled study | 2010–2011 | -Intersphincteric resection; anterior resection or Miles resection of rectal cancer stage I–III -Rectal cancer verified with coloscopy -Consent to surgery | -Acute inflammatory or infectious disease -Bowel obstruction such as ileus -Bowel perforation or fever -Tumour recurrence or stage IV -No consent | Preoperative and postoperative Solu-Medrol treatment (0.4 mg/kg once daily from 5 d before surgery to 5 d after surgery) (50) | Placebo, administration identical with exposure regime (50) | |
| Huang et al (2018) [23] | Retrospective cohort study | 2006–2009 | -Lung resection or lobectomy of NSCLC -Above 18 years -NSCLC confirmed by postoperative pathology | -Primary malignant tumour in other place (15) -Recurrent metastatic lung tumour -Long-term steroid exposure -Impossible follow-up because of missing data (4) | Perioperative dexamethasone treatment (2–15 mg) (332) | No perioperative dexamethasone treatment (256) | OS, DFS |
| Kim et al (2019) [24] | Retrospective cohort study | 2005–2010 | -Breast conserving surgery or mastectomy for breast cancer stage I–III | -Multiple surgeries simultaneously (63) -Lack of anaesthetic or surgical information (21) - Steroid therapy for any reason (17) | Perioperative single dose of IV dexamethasone treatment (236) | No perioperative single dose of IV dexamethasone treatment (2392) | Recurrence, OS |
| Lazzara et al (2018) [25] | Retrospective cohort study | 2012–2016 | -Curative resection in adults with histologically proven stage I-III colorectal cancer | -Chronic inflammatory disease including bowel disease (IBD) -Long-term immunosuppressive therapy -Carcinoma in situ (intraepithelial or invasion of lamina propria) -Cancer infiltrating an adjacent organ or with radiological or surgical evidence of metastasis -Missing records of preoperative corticosteroids | Preoperative oral prednisone treatment (50 mg 13, 7 and 1 hour before surgery, totalling 150 mg) for all allergic patients (61) | No preoperative oral prednisone treatment (188) | OS, DFS |
| Merk et al (2016) [26] | Retrospective cohort study | 2003–2007 | -Surgery for endometrial cancer stage I–IV | - | Dexamethasone treatment (single dose 4–10 mg) (107) | No single dose dexamethasone treatment (202) | Recurrence, OS, DFS |
| Okazumi et al (2004) [27] | Retrospective cohort study | 1995–1999 | -Resection with three-field lymphadenectomy of the neck, mediastinum and abdomen of oesophageal squamous cell carcinoma stage 0–IVa | -Chemoradiotherapy before surgery (85) | Intraoperative methylprednisolone treatment (250 mg) and postoperative methylprednisolone treatment (125 mg) POD1 and POD2 (19) | No intraoperative methylprednisolone treatment (18) | OS |
| Sandini et al (2018) [28] | Retrospective cohort study | 2007–2015 | -Pancreaticoduodenectomy for pancreatic ductal adenocarcinoma | -Incomplete anaesthesia records (<2% of the cohort) -Invasive carcinoma from intraductal papillary mucinous neoplasms | Intraoperative dexamethasone (4–10 mg) (117) | No intraoperative dexamethasone (562) | OS |
| Sato et al (2002) [29] | Double blinded randomized controlled study | 1996–1999 | -Resection of oesophageal squamous cell carcinoma stage I–III | -Preoperative chemotherapy, radiation- or immunotherapy -Age over 76 years -Preoperative complications (such as liver cirrhosis, IDDM, CC < 60 mL/min, VC < 80%, FEV1 < 70%) -HBs-antigen or HCV-antibody positive -Multiple cancer -Old tuberculosis lesions | Preoperative methylprednisolone treatment (10 mg/kg body weight diluted in 100 mL physiologic saline within 30 minutes of the start of the surgery) (33) | A corresponding placebo infusion (33) | OS |
| Shimada et al (2004) [30] | Retrospective cohort study | 1993–2000 | -Radical esophagectomy for primary thoracic oesophageal squamous cell carcinoma stage I–IV | -Any preoperative adjuvant therapy | Intraoperative methylprednisolone treatment (250 mg) and postoperative methylprednisolone treatment (125 mg) POD1 and POD2 (78) | No intraoperative methylprednisolone treatment (63) | OS, CSS |
| Singh et al (2014) [31] | Follow up analysis of a previous double blinded randomized clinical trial | 2006–2008 | -Hemicolectomy for colon cancer stage I–III | -Immunosuppressive therapy including steroids -ASA score IV or V -Requirement of stoma -Unable to speak English -Significant cognitive impairment -Stage IV disease at time of surgery (3) | Preoperative dexamethasone treatment (8 mg at least 90 min before incision) (20) | Saline placebo at least 90 minutes before incision (23) | OS, DFS |
| Yano et al (2005) [32] | Double-blinded randomized controlled study | 1997–1999 | -Esophagectomy for thoracic oesophageal cancer | - | Preoperative methylprednisolone drip infusion (500 mg/body in saline 2 h preoperatively) (20) | Saline placebo 2 hours preoperatively (20) | Recurrence, OS, DFS |
| Yu et al (2015) [33] | Retrospective cohort study | 2007–2011 | -Curative resection of rectal cancer stage I–III -Histologically proven adenocarcinoma less than 15 cm from the anal verge | -Immunosuppressive therapy including recent steroid use (2) -Chronic inflammatory disease (including IBD) (5) -FAP diagnosis (2) -Multiple primary cancer (4) -Incomprehensive prescription records | Postoperative and/or intraoperative IV dexamethasone treatment (4–10 mg) (75) | No postoperative and/or intraoperative dexamethasone treatment (440) | OS, DFS |
| Zhu et al (2017) [34] | Retrospective cohort study | 2003–2005 | -Curative lung cancer surgery -No neoadjuvant therapy | -Other primary tumour(s) -Recurrence of previous lung cancer -Long-term steroid treatment -Missing records | Intraoperative dexamethasone treatment (94) | No intraoperative dexamethasone treatment (209) | DFS |
| Study (Publication Year) | n | Follow-Up, Years | Age | Gender M (%) F (%) | ||
|---|---|---|---|---|---|---|
| Glucocorticoid | Control | Glucocorticoid | Control | |||
| Call et al (2015) [19] | 144 | 1.2A | 65A | 67 A | 33 (47.8) 36 (52.2) | 43 (57.3) 32 (62.7) |
| Cata et al (2016) [20] | 1549 | 1–12 | 65.4 C | 63.5 C | 176 (40.1) 263 (59.9) | 603 (54.3) 507 (45.7) |
| De Oliveira et al (2014) [21] | 260 | 4–10 | 57 A | 58 A | 0 (0.0) 102 (100) | 0 (0.0) 158 (100) |
| Gan et al (2015) [22] | 100 | 3.75 B | 53.2 B | 50.1 B | 24 (48) 26 (52) | 28 (56) 22 (44) |
| Huang et al (2018) [23] | 588 | 6–10 | NA | NA | 374 (63.6) * 214 (36.4) * | |
| Kim et al (2019) [24] | 2628 | 5–10 | 49.5 B | 50.1 B | 0 (0.0) 236 (100) | 0 (0.0) 2392 (100) |
| Lazzara et al (2018) [25] | 249 | 0–4.75 | 66 A | 69 A | 26 (42.6) 35 (57.4) | 110 (58.5) 78 (41.48) |
| Merk et al (2016) [26] | 309 | 4.3 (G) A 3.9 (C) A | 64 A | 66 A | 0 (0.0) 107 (100) | 0 (0.0) 202 (100) |
| Okazumi et al (2004) [27] | 37 | 3–8 | 61 B | 62 B | 18 (94.7) 1 (5.3) | 16 (88.9) 2 (0.1) |
| Sandini et al (2018) [28] | 679 | 2 A | 65 A | 67 A | 38 (32.5) 79 (67.5) | 284 (50.5) 278 (49.5) |
| Sato et al (2002) [29] | 66 | 1.5–4.5 | 62 B | 64 B | 29 (88) 4 (12) | 31 (94) 2 (6) |
| Shimada et al (2004) [30] | 141 | 3-12 | 64 A | 64 A | 66 (84.6) 12 (15.4) | 55 (87.3) 8 (12.7) |
| Singh et al (2014) [31] | 43 | 4.8–6.5 | 72 A | 71 A | 6 (30.0) 14 (70.0) | 13(56.5) 10(43.5) |
| Yano et al (2005) [32] | 40 | 5 | 63.5 B | 55.9 B | 17 (85.0) 3 (15.0) | 19 (95.0) 1 (5.0) |
| Yu et al (2015) [33] | 515 | 3–7 | 60 A | 59 A | 47 (63) 28 (37) | 250 (57) 190 (43) |
| Zhu et al (2017) [34] | 303 | 2.5–8.45 | 61 A,* | 101 (33.3) * 202 (66.7) * | ||
| a. The Newcastle-Ottawa Scale for Non-Randomised Studies | ||||||
| Study (Publication Year) | Selection (Max 4 Stars) | Comparability (Max 2 Stars) | Outcome (Max 3 Stars) Loss to Follow up <10% Earns a Star if Unlikely to Introduce Bias | Total (Max 9 Stars) | ||
| Call et al (2015) [19] | *** | ** | ** | 7 | ||
| Cata et al (2016) [20] | *** | ** | ** | 7 | ||
| De Oliveira et al (2014) [21] | **** | ** | ** | 8 | ||
| Huang et al (2018) [23] | *** | ** | ** | 7 | ||
| Kim et al (2019) [24] | *** | ** | ** | 7 | ||
| Lazzara et al (2018) [25] | *** | * | 4 | |||
| Merk et al (2016) [26] | *** | ** | *** | 8 | ||
| Okazumi et al (2004) [27] | *** | * | 4 | |||
| Sandini et al (2018) [28] | *** | * | ** | 6 | ||
| Shimada et al (2004) [30] | ** | * | *** | 6 | ||
| Yu et al (2015) [33] | **** | ** | ** | 8 | ||
| Zhu et al (2017) [34] | *** | ** | ** | 7 | ||
| b. The Cochrane Risk of Bias Evaluation 1.0 for Randomised Studies | ||||||
| Study (Publication Year) | Selection Bias | Performance Bias | Detection Bias | Attrition Bias | Reporting Bias | |
| Sequence Generation | Allocation Concealment | Blinding of participants and Personnel | Blinding of Outcome Assessors | Incomplete Outcome Data | Selective Outcome Reporting | |
| Yano et al (2005) [32] | Unclear risk of bias | Unclear risk of bias | Unclear risk of bias | Unclear risk of bias | Unclear risk of bias | Low risk of bias |
| Singh et al (2015) [31] | Low risk of bias | Low risk of bias | Low risk of bias | Unclear risk of bias | Low risk of bias | Low risk of bias |
| Sato et al (2002) [29] | Low risk of bias | Low risk of bias | Low risk of bias | Unclear risk of bias | Low risk of bias | Low risk of bias |
| Gan et al (2015) [22] | Low risk of bias | Low risk of bias | Low risk of bias | Low risk of bias | Low risk of bias | Low risk of bias |
| Outcome | Effect Estimate | 95% CI | p-Value | Prediction Interval | I2 |
|---|---|---|---|---|---|
| Recurrence (non-randomised studies) | |||||
| 1-year (RR) | 1.01 | 0.78–1.31 | 0.93 | - | 0% |
| 3-year (RR) | 1.00 | 0.85–1.18 | 0.97 | - | 0% |
| 5-year (RR) | 1.04 | 0.87–1.25 | 0.67 | - | 21% |
| Time-to-event (HR) | 1.18 | 0.78–1.79 | 0.32 | 0.42–3.35 | 9% |
| Overall survival (randomised studies) | |||||
| 1-year (RR) | 1.20 | 0.55–2.60 | 0.65 | - | 0% |
| 3-year (RR) | 1.09 | 0,70–1.70 | 0.69 | - | 0% |
| 5-year (RR) | 1.64 | 1.00–2.71 | 0.05 | - | 0% |
| Time-to-event (HR) | 1.46 | 0.61–3.46 | 0.20 | 0.07–30.4 | 0% |
| Overall survival (non-randomised studies) | |||||
| 1-year (RR) | 0.70 * | 0.51–0.97 | 0.03 | - | 29% |
| 3-year (RR) | 0.89 | 0.71–1.13 | 0.34 | - | 68% |
| 5-year (RR) | 1.02 | 0.84–1.25 | 0.81 | - | 80% |
| Time-to-event (HR) | 0.98 | 0.75–1.27 | 0.86 | 0.44–2.19 | 60% |
| Disease-free survival (randomised studies) | |||||
| 1-year (RR) | 1.34 | 0.37–4.83 | 0.65 | - | 14% |
| 3-year (RR) | 1.31 | 0.74–2.31 | 0.35 | - | 0% |
| 5-year (RR) | 1.54 | 0.94–2.53 | 0.09 | - | 0% |
| Disease-free survival (non-randomised studies) | |||||
| 1-year (RR) | 0.77 * | 0.60–0.97 | 0.03 | - | 0% |
| 3-year (RR) | 1.08 | 0.78–1.51 | 0.64 | - | 79% |
| 5-year (RR) | 1.11 | 0.74–1.67 | 0.61 | - | 93% |
| Time-to-event (HR) | 1.03 | 0.65–1.62 | 0.88 | 0.31–3.36 | 84% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosenkrantz Hölmich, E.; Petring Hasselager, R.; Tvilling Madsen, M.; Orhan, A.; Gögenur, I. Long-Term Outcomes after Use of Perioperative Glucocorticoids in Patients Undergoing Cancer Surgery: A Systematic Review and Meta-Analysis. Cancers 2020, 12, 76. https://doi.org/10.3390/cancers12010076
Rosenkrantz Hölmich E, Petring Hasselager R, Tvilling Madsen M, Orhan A, Gögenur I. Long-Term Outcomes after Use of Perioperative Glucocorticoids in Patients Undergoing Cancer Surgery: A Systematic Review and Meta-Analysis. Cancers. 2020; 12(1):76. https://doi.org/10.3390/cancers12010076
Chicago/Turabian StyleRosenkrantz Hölmich, Emma, Rune Petring Hasselager, Michael Tvilling Madsen, Adile Orhan, and Ismail Gögenur. 2020. "Long-Term Outcomes after Use of Perioperative Glucocorticoids in Patients Undergoing Cancer Surgery: A Systematic Review and Meta-Analysis" Cancers 12, no. 1: 76. https://doi.org/10.3390/cancers12010076