-
PDF
- Split View
-
Views
-
Cite
Cite
S. Meran, K. Don, N. Shah, K. Donovan, S. Riley, A.O. Phillips, Impact of chronic kidney disease management in primary care, QJM: An International Journal of Medicine, Volume 104, Issue 1, January 2011, Pages 27–34, https://doi.org/10.1093/qjmed/hcq151
Close - Share Icon Share
Abstract
Background: The introduction of eGFR reporting and publication of national CKD guidelines has led to major challenges in primary and secondary care, leading to an increase in the number of referrals to nephrology clinics. We have shown that introduction of a renal patient care pathway reduces nephrology referrals and enables managed discharges of CKD patients to primary care. The aim of this article is to examine the outcome of patients discharged to primary care to find out if there is an associated risk with increased discharge supported by the patient pathway.
Methods: The study was carried out within a single NHS Trust covering a population of 560 000. All patients discharged from the trust’s renal outpatient clinic between June 2007 and July 2008 were identified. Patient notes and the local laboratory database systems were used to determine the source and timing of tests.
Results: A total of 31 new referrals and 57 regular follow-ups were discharged during this period. The median age of discharge was 67.5 years. Most subjects (60%) had CKD stage 3 at the time of discharge. A total of 23% of discharges were categorized as CKD stages 1, 2 or normal and 17% of patients had CKD stage 4. Overall, 93% had stable eGFRs prior to discharge, 77.5% of patients had blood pressure within threshold (140/90 according to UK CKD guidelines) and 97.7% of patients had haemoglobins >10 g/dl. Post-discharge 83% of patients had eGFRs recorded by their general practitioner and 92.6% of these were measured within appropriate time frames as per CKD guidelines. The majority of patients (82%) had either improved or stable eGFR post-discharge and only three patients had a significant decline in their eGFR.
Conclusions: These data indicate that selected CKD patients can be appropriately discharged from secondary care and adequately monitored in primary care. Furthermore, we have shown that this was a safe practice for patients.
Introduction
Chronic kidney disease (CKD) has now been recognized as a major public health concern. The realization that it is a potent risk factor for cardiovascular disease and its inclusion in the quality and outcomes framework (QOF) of the general practitioner (GP) contract in the UK, has resulted in a shift in CKD management from the domain of secondary care into the realms of preventative medicine in primary care.
This change in practice has meant that physicians who have previously had little or no experience in managing renal disease are now principle providers of care for these patients. It is essential, therefore, that Nephrology Units establish an easily accessible means for providing support and advice to Primary Care Services. In South East Wales, we have addressed this by the introduction of a Map of Medicine based renal patient care pathway.1 This is a web-based facility available to all Primary Care Trusts within South East Wales and provides evidence-based patient care pathways covering 28 medical specialities. The renal pathway combines the CKD guidelines and recommendations of the Welsh National Service Framework to provide clear guidance on appropriate referral to nephrology services. In addition, it provides support and education to enable the management of stable uncomplicated renal patients in primary care.
In April 2006, reporting of estimated glomerular filtration rate (eGFR) based on the modification of diet for renal disease (MDRD) formula was implemented nationwide. In our previous article we described the effect of eGFR reporting and the introduction of CKD into the GP QOF in South East Wales before and after the introduction of the Map of Medicine to primary care trusts.2 We showed a rapid increase in the number of referrals to nephrology clinics following introduction of eGFR and CKD QOF. Subsequent introduction of the renal Map of Medicine, however, led to a fall in the number of referrals and enabled managed discharges of CKD patients with a perceived low risk of progression from secondary to primary care. Specifically, the patient care pathway resulted in earlier discharge of new referrals and a doubling of the number of follow-up patients discharged from secondary care, thereby increasing capacity to accommodate more complex nephrology referrals within current resources. However, it has not yet been ascertained whether this change in healthcare practice is low-risk for patients. In this article we aim to determine if patients discharged from nephrology clinic were appropriately discharged and adequately monitored in primary care. In addition, we prospectively follow-up the discharged patients’ renal parameters and determine whether they were appropriately re-referred to nephrology services.
Methods
The study was carried out within a single NHS Trust in South East Wales. The Trust covers five local health boards and has a population catchment of 560 000. Using clinic notes and electronic patient records, all patients discharged from the trusts renal outpatient clinic between June 2007 and July 2008 were identified. None of the patients seen in this clinic are on renal replacement therapy (RRT), and any patients discharged from the clinic to RRT were excluded. Patients who were discharged due to failure to attend three consecutive clinic appointments were also excluded.
The patients were categorized into two groups: new referrals from GPs that were discharged back to primary care, and those patients with stable CKD that were already under secondary care follow-up and were discharged to primary care. The vast majority of new referrals that were discharged were seen only once in nephrology clinic prior to discharge (97%), with the remaining 3% having a maximum of three consultations. A single consultant nephrologist performed triaging of new referrals, and new referrals that were inappropriate or did not have sufficient information on the referral letter were sent back to the GP for further clarification.
Information on patient age, sex, primary renal diagnosis and date of referral to and discharge from nephrology outpatient clinic were collected from patient notes. Information on other co-morbidities such as diabetes, hypertension, ischaemic heart disease, peripheral vascular disease, cerebrovascular disease and malignancy were also simultaneously collected.
Using patient records, the renal database (PROTON) and the local laboratory system, each patient’s eGFR, haemoglobin, blood pressure (BP) and urinalysis results at the point of discharge from clinic were recorded. eGFR post-discharge, and the timing and source (GP or hospital) of these tests were also recorded from our local laboratory database systems—all blood samples taken from primary care practices involved are sent to the trusts laboratory for analysis. eGFR was calculated by the local laboratory system using the MDRD formula. The calculated eGFR was then used to categorize patients into their CKD stages at discharge. The calculated eGFR and CKD stage post-discharge were subsequently used to determine which patients should have been re-referred to nephrology clinic according to the current CKD guidelines.3 In addition, patients re-referred by GPs to nephrology clinic were identified; and the appropriateness of re-referrals were assessed. All the recorded data was collected in early 2009 and compiled into a database using Microsoft Access for analysis.
Results
Patient characteristics
During the 12-month period between June 2007 and July 2008, there were a total of 103 discharges from the trusts outpatient nephrology clinic. Seven of these patients were excluded as they failed to attend three clinic appointments. A further eight patients were excluded as they were discharged from the clinic to RRT. A total number of 88 patients were therefore used for the analyses. Any patient whose death occurred subsequent to discharge was included in the analysis.
The median age of the patients at discharge was 67.5 years (range 18–94 years), with 47 (53.4%) of the patients being male and 41 (46.6%) patients being female. There were 31 new patients discharged (35.2%) and their median age was 68 years. There were 57 patients (64.6%) discharged in the ‘follow-up’ group. Their median age was 67 years and the average follow-up period was 1084 days (155 weeks). The median waiting time for a new patient appointment at the time of the study was 7 months.
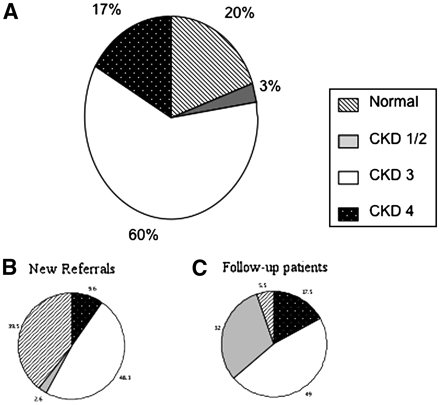
Table 1 shows the percentage of discharged patients with additional co-morbidities, with diabetes and hypertension being the most common. The CKD stage distribution of all the discharged patients is shown in Figure 1A. A large majority (60%) of the patients had CKD stage 3. A total of 23% of the patients were either labelled as normal or had CKD stages 1 and 2, and 17% of patients had CKD stage 4. Figure 1B and C shows the CKD stage distribution of the new and follow-up groups respectively. They show that the majority of new referrals that were discharged were either diagnosed as having normal renal function (39.5%) or were identified as CKD stage 3 (48.3%). The patients labelled as having normal renal function on discharge were mostly new referrals to the clinic. The majority of these had been referred with eGFRs between 60 and 90 ml/min, with no structural renal abnormality or no abnormality detected on urine dipstick. A small proportion of these patients were referred with abnormal eGFR’s that subsequently improved. The figures also show that most of the CKD stage 4 patients that were discharged were follow-up patients.
Demographics: numbers and percentages of discharged patients with the given additional co-morbidities.
| . | Patient numbers . | Percentage . |
|---|---|---|
. | ||
| DM | 23 | 26.1 |
| IHD | 14 | 15.9 |
| Hypertension | 32 | 36.4 |
| CVD | 6 | 6.8 |
| Malignancy | 0 | 0 |
| PVD | 1 | 1.1 |
| . | Patient numbers . | Percentage . |
|---|---|---|
. | ||
| DM | 23 | 26.1 |
| IHD | 14 | 15.9 |
| Hypertension | 32 | 36.4 |
| CVD | 6 | 6.8 |
| Malignancy | 0 | 0 |
| PVD | 1 | 1.1 |
DM (diabetes), IHD (ischaemic heart disease), CVD (cerebrovascular disease), PVD (peripheral vascular disease).
Demographics: numbers and percentages of discharged patients with the given additional co-morbidities.
| . | Patient numbers . | Percentage . |
|---|---|---|
. | ||
| DM | 23 | 26.1 |
| IHD | 14 | 15.9 |
| Hypertension | 32 | 36.4 |
| CVD | 6 | 6.8 |
| Malignancy | 0 | 0 |
| PVD | 1 | 1.1 |
| . | Patient numbers . | Percentage . |
|---|---|---|
. | ||
| DM | 23 | 26.1 |
| IHD | 14 | 15.9 |
| Hypertension | 32 | 36.4 |
| CVD | 6 | 6.8 |
| Malignancy | 0 | 0 |
| PVD | 1 | 1.1 |
DM (diabetes), IHD (ischaemic heart disease), CVD (cerebrovascular disease), PVD (peripheral vascular disease).
CKD stage of patients at the point of discharge shown as percentage. (A) The CKD stage for all patients. (B)The CKD stage distribution for only the new referrals and (C) only for follow-up patients at discharge.
Appropriate discharge from secondary care
Renal parameters
Of the 88 discharged patients, 87 (98.9%) had eGFRs recorded on the day of discharge from clinic. Analysis of the ‘follow-up’ group showed that the 93% (53 out of 57 patients) had documented stable eGFRs prior to discharge. The majority of these patients (42%) had stable eGFR’s for >5 years prior to discharge. A large proportion (28%) had stable eGFRs for >2 years, with a further 23% having stable eGFRs for >1-year period prior to discharge. All patients that were discharged with CKD Stage 4 demonstrated stable renal function prior to discharge.
Of 88 patients, 78 (88.6%) had recorded urinalyses at the time of discharge. Only four patients were identified with significant proteinuria (defined as having >3+ protein on dipstick testing). Subsequent analysis of these four patients showed that one of these patients was discharged to a nephrology clinic at another trust. One patient requested to be seen by a private nephrologist. One had multiple co-morbidities and died with a stable eGFR. No information was available on the fourth patient.
BP on discharge
According to the UK CKD guidelines the target BP for CKD patients is 130/80 (125/75 if proteinuria), with a threshold BP of 140/90.3 In our study, 80 patients (91%) had a BP recorded in the notes at their last consultation. The median systolic BP at discharge was 133 mmHg (range 97–169 mmHg) and median diastolic BP was 73 mmHg (range 54–94 mmHg). In total, 52.5% of patients had a BP within target at discharge and 77.5% of patients had a BP within threshold.
Haemoglobin at discharge
A total of 86 patients (97.7%) had recorded haemoglobins at the time of discharge. The mean haemoglobin at discharge was 12.3 g/dl. Only 6.8% of patients had haemoglobins <11 g/dl, with 97.7% of patients having haemoglobins >10 g/dl.
Appropriate renal monitoring in primary care
A total of 83% of discharged patients had eGFRs recorded post-discharge. Of these 82.2% were taken by the GP, whilst 17.8% were either recorded by the hospital or the source of the test was unknown. A total of 15 patients had no measure of eGFR post-discharge. Six of these patients had normal renal function at discharge, one had stable CKD stage 2 and six had stable CKD stage 3. The remaining two patients had stable CKD stage 4 prior to discharge. One of these patients was 81 years of age with documented cerebrovascular disease. The other patient was subsequently admitted to hospital with dilated cardiomyopathy and died.
The guidelines state that if stable, CKD stages 1 and 2 should be monitored annually, CKD stage 3 6-monthly and stage 4 3-monthly.3 In our analysis the large majority of patients had eGFR’s recorded within appropriate time frames. Overall, 92.6% of patients had timely eGFR measurements post-discharge and 83.3% of the eGFR measurements were performed by primary care trusts rather than by other hospital-based specialities.
Patient outcomes
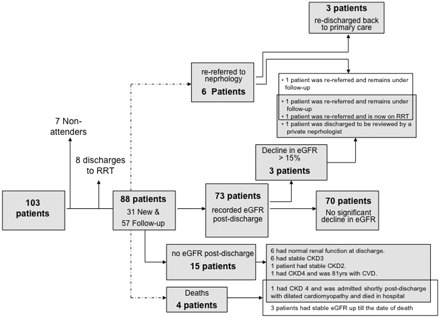
A simplified diagram showing patients renal outcomes, re-referral to nephrology clinic and deaths is illustrated in Figure 2.
Diagrammatic illustration of patient outcomes post discharge from nephrology clinic. The overlapping boxes indicate patients belonging to more than one group.
Renal function
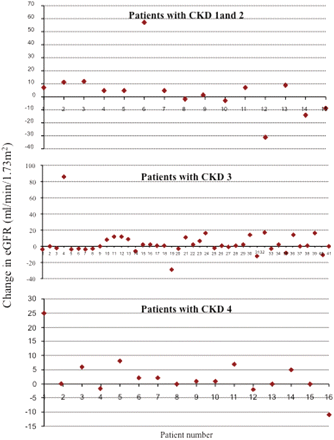
The UK CKD guidelines define a significant decline in renal function as a decline in eGFR of greater than 15%.3 In our study, 73 patients had eGFR’s measured post-discharge. Of these patients 60 patients (82%) were found to have either stable or improved eGFRs. In Figure 3, the change in eGFR for each individual patient is plotted, with a negative number implying a fall in eGFR. The graphs demonstrate that in each CKD stage, more patients had a rise in eGFR rather than a fall. The graphs also show that only three patients overall had a significant decline in their eGFR and that these appeared to be equally distributed between the different CKD stages. Detailed examination of the data confirmed that only these three patients had a decline of >15% in their eGFR. Further analysis showed that one of these patients was re-referred and remains under follow-up, one patient was re-referred and is now on dialysis and one patient was discharged to be reviewed by a private nephrologist at their request.
Change in eGFR post discharge for each individual patient divided according to CKD stage. A negative number implies a fall in eGFR (n = –73).
Re-referrals
Of the 88 patients included in the analysis, six patients were re-referred back to nephrology clinic following discharge. Three of these patients were rapidly re-discharged back to primary care, two remain under nephrology follow-up and one patient is now on dialysis. The patient who is now on dialysis was a new referral. He was an 85-year-old male with a diagnosis of renovascular disease, and had diabetes and hypertension as co-morbid conditions. At the point of discharge he had no haematuria or proteinuria and his blood pressure was well controlled. He was promptly re-referred to nephrology services following a decline in eGFR and started on dialysis in a timely manner.
Patient deaths
There were four deaths in total. Three of these patients had stable CKD stage 4 at the point of discharge and one had stable CKD stage 3. Post-discharge, three of the patient’s eGFR’s remained stable until the date of death. The fourth patient showed a rapid deterioration in eGFR following admission to hospital with dilated cardiomyopathy.
Discussion
The global epidemic of type 2 diabetes, along with a rise in the proportion of people with obesity and hypertension, in our largely ageing populations has resulted in an alarming increase in the prevalence of CKD worldwide.4–7 Moreover, in 2002 the National Kidney Foundation of the US proposed guidelines through the Kidney Disease Outcomes Quality Initiative (K/DOQI) program that classified CKD into five stages based on the eGFR.8 This system has provided a standardized means of estimating the prevalence of CKD, and further facilitated awareness of the extent of this condition within our populations. Together, both these factors have led to what has been described as an ‘epidemic of CKD’.
In the UK, the 2005 New Opportunities for Early Renal Intervention by Computerised Assessment Study (NeoErica) showed a 4.9% prevalence of CKD in the general population, estimating that 1 in 200 people suffer from this condition.9 Highlighting the issue, the second part of the National Service Framework for renal services in the UK was published in 2005 focussing on early identification, management and prevention of progression of CKD.10 Subsequently CKD was also included in the QOF of the GP contract in 2006, providing financial incentives to encourage continual improvements in the clinical care of CKD patients. These strategies aimed to enhance the early identification of patients with CKD, allowing for appropriate measures to be taken to slow/prevent its progression and to address the potential complications of CKD. In addition, early detection also enables timely referral to secondary care services, facilitating patient management into various renal care pathways in preparation for transplantation, RRT or conservative therapy.
The UK CKD guidelines were subsequently published to provide primary and secondary care services comprehensive advice on all aspects of CKD management.3 The guidelines endorse the management of selected CKD patients in primary care and promote the provision of an easily accessible non-visit based specialist advice service for GPs. In South East Wales, we have achieved this by the introduction of the renal Map of Medicine to primary care services. This was supported by clear management plans in clinic letters to GPs and educational ‘CKD roadshows’ for primary care trusts. One of the aims of the renal patient pathway was to facilitate discharge of patients from nephrology clinic to primary care. In our previous article we demonstrated that introduction of this pathway enabled earlier and greater discharge of patients to primary care.2 However, although we have successfully implemented a strategy for discharge and management of CKD patients in primary care in our area, the consequences of this change in practice in terms of patient’s safety were previously not verified. In this article we have shown that the management of stable CKD patients in primary care is a safe practice, and that in the absence of proteinuria and uncontrolled BP these patients are unlikely to develop progressive renal impairment.
The UK CKD guidelines state that all patients with CKD stages 4 and 5 should be referred to or discussed with specialist nephrology services.3 Our data shows that the majority of analysed patients were discharged appropriately having either normal renal function or having CKD stages 1, 2 or 3. In addition, all the follow-up patients, with the exception of four people, had stable renal function for at least one year prior to discharge. A significant proportion of the follow-up patients had CKD stage 4. However, it is recognized that not all patients with CKD stage 4 necessarily progress to end-stage renal failure. Reassuringly, all the patients that were discharged with CKD stage 4 were identified as being stable prior to discharge. In our analysis, three of these patients died post-discharge. However, two of these patients had stable renal function at the time of death, and the third patient’s death was not related to their renal impairment; highlighting that, especially in the elderly, CKD is a greater risk factor for death from cardiovascular disease than from end-stage renal disease. Analysis of urine and BP at the point of discharge also indicated that the patients were appropriately selected for discharge. Although our analysis shows that only 98.9% had eGFR’s recorded, 88.6% had urinalysis recorded and 91% had BPs recorded, these percentages only relate to measurements recorded on the day of discharge. It should be noted that all our patients would have had these parameters routinely measured regularly either during their follow-up period or prior to referral from GP.
The assumption that primary care services would appropriately monitor CKD patients and correctly re-refer suitable patients back to nephrology services was also substantiated. This study confirms that the large majority of patients had sufficient blood analysis performed within a timely manner following discharge from nephrology clinic. Importantly, the data also shows that the CKD patients monitored in primary care on the whole had either stable or improved renal function suggesting that GPs are providing a good standard of care for these patients. It is worth noting here, however, that the tendency of eGFR to rise after discharge is most likely explained by a regression to the mean, with patients being referred at (or soon after) a sudden decline in eGFR. The patients who had a significant decline in their renal function were equally distributed among the different CKD stages, suggesting that the decline in eGFR may not have been predictable. Crucially, the data also shows that the patients were almost entirely appropriately re-referred to nephrology services when required, suggesting that the practice is low-risk for patients.
The risk of developing CKD increases with increasing age and, as expected, the median age of the patients in this study was >65 years of age. Within primary care there has been concern that the introduction of eGFR reporting and the development of primary care CKD registers will lead to possible overburden of services by this new cohort of elderly patients that require input and monitoring. However, as demonstrated in our analysis, a considerable proportion of CKD patients are also likely to have other chronic illnesses such as diabetes, hypertension and ischaemic vascular disease. It is expected, therefore, that these patients are already registered with their primary care physician for the management of their cardiovascular risk factors. Since this is an important aspect of CKD patient management, monitoring of these patients in primary care will not necessarily mean uptake of a new cohort of patients, but rather a slightly alternative approach to an existing cohort of patients. This will also provide greater continuity of care for these patients, avoiding unnecessary blood tests and confusion with duplicate advice from secondary care.
Whilst the data from this study is encouraging, it was not a randomized comparison and longer-term patient outcomes have not been assessed. Furthermore, a CKD management program in primary care should also encompass control of BP, reduction of proteinuria, treatment of hyperlipidaemia, smoking cessation advice and the treatment of metabolic bone disease. However, the procurement of data from primary care trusts to establish monitoring and management of all of these parameters could not be established for this analysis and should be the subject of future studies. Nevertheless, this study does conclusively demonstrate that primary care based management of CKD patients does not have a detrimental effect on renal outcomes. This data is consistent with another UK study performed in Scotland, and indicates that the quality of CKD patient management in primary care is not subject to regional variation.11 This will allow us to roll out the program to other nephrology clinics and primary care trusts within Wales. In comparison to the study performed in Scotland there was a larger cohort of patients discharged with CDK stage 4 in this study, thus indicating that advanced stable CKD is effectively managed in primary care. Moreover whilst the study performed in Scotland also indicates that patients can be appropriately monitored in primary care, this study goes further and examines the renal outcomes of the discharged patients thereby highlighting the impact of discharge on renal parameters.
In summary, this study has demonstrated that with the aid of a renal patient care pathway, patients with stable CKD can be appropriately discharged from secondary care and adequately monitored in primary care. Furthermore, follow-up of these patients primary care revealed that this was associated with low-risk in terms of decline in renal function. This study suggests that primary care based management in this form is an effective method of managing selected patients with CKD in the UK.
Conflict of interest: None declared.