Abstract
Background:
Contextual socio-economic factors, health-care access, and general practitioner (GP) involvement may influence colonoscopy uptake and its timing after positive faecal occult blood testing (FOBT). Our objectives were to identify predictors of delayed or no colonoscopy and to assess the role for GPs in colonoscopy uptake.
Methods:
We included all residents of a French district with positive FOBTs (n=2369) during one of the two screening rounds (2007–2010). Multilevel logistic regression analysis was performed to identify individual and area-level predictors of delayed colonoscopy, no colonoscopy, and no information on colonoscopy.
Results:
A total of 998 (45.2%) individuals underwent early, 989 (44.8%) delayed, and 102 (4.6%) no colonoscopy; no information was available for 119 (5.4%) individuals. Delayed colonoscopy was independently associated with first FOBT (odds ratio, (OR)), 1.61; 95% confidence interval ((95% CI), 1.16–2.25); and no colonoscopy and no information with first FOBT (OR, 2.01; 95% CI, 1.02–3.97), FOBT kit not received from the GP (OR, 2.29; 95% CI, 1.67–3.14), and socio-economically deprived area (OR, 3.17; 95% CI, 1.98–5.08). Colonoscopy uptake varied significantly across GPs (P=0.01).
Conclusion:
Socio-economic factors, GP-related factors, and history of previous FOBT influenced colonoscopy uptake after a positive FOBT. Interventions should target GPs and individuals performing their first screening FOBT and/or living in socio-economically deprived areas.
Similar content being viewed by others
Main
Colorectal cancer (CRC) is the fourth leading cause of cancer death worldwide (Ferley et al, 2010). Studies show that population-based CRC screening programmes involving biennial faecal occult blood tests (FOBTs) followed by colonoscopy if positive decrease CRC mortality by 15% to 20% when the participation rate is at least 50% (Hewitson et al, 2008). Although a positive FOBT requires further evaluation by colonoscopy, no guidelines about the optimal timing of colonoscopy after a positive FOBT are available. However, the Veterans Health Administration Directive and Canadian Association of Gastroenterology Wait Time Consensus Group recommend colonoscopy within 60 calendar days of the positive FOBT (Paterson et al, 2006; VHA directive, 2007). In one study, longer time-to-colonoscopy was an independent risk factor for adenomas and showed trends towards associations with advanced adenomas and invasive CRC (Gellad et al, 2009).
Age, gender, absence of health insurance, physician decisions, non-white ethnicity, rurality, socio-economic deprivation of the area of residence, and greater distance to regional capital have been shown to be associated with colonoscopy uptake after a positive FOBT (Turner et al, 2003; Etzioni et al, 2006; Fisher et al, 2006; Rao et al, 2009; Steele et al, 2010; Dupont-Lucas et al, 2011; Morris et al, 2012; Moss et al, 2012). However, discordant results were observed and only one study investigated factors associated with time from positive FOBT to colonoscopy (Dupont-Lucas et al, 2011).
Furthermore, no studies used multilevel modelling, which takes into account the hierarchical data structure and is more suitable for investigating the impact on colonoscopy uptake of contextual effects such as socio-economic deprivation and health-care access inequalities. (Chaix and Chauvin, 2002; Pornet et al, 2010; Le Breton et al, 2012).
Given the specific role played by general practitioners (GPs) in informing patients about screening protocols and encouraging patient adherence to colonoscopy after a positive FOBT, greater GP involvement might be associated with higher levels of colonoscopy uptake.
We previously found that participation in CRC screening was significantly lower in males, the youngest age group (50–59 years), and patients living in socio-economically deprived areas (Le Breton et al, 2012), in keeping with other studies (Weller et al, 2007; Frederiksen et al, 2010; Pornet et al, 2010; Moss et al, 2012).
Our primary objective was to identify both individual and contextual factors associated with delayed or no colonoscopy in individuals who had positive results from an FOBT performed as part of a population-based screening programme. We also assessed the role of GPs on colonoscopy uptake.
Materials and methods
Colorectal cancer screening programme
This retrospective cohort study was conducted in France in the Val-de-Marne district, a Paris suburb with a population of 1.3 million (2% of the French population). The local screening programme centre (Association de dépistage organisé des cancers dans le Val-de-Marne) mails letters to all individuals aged 50–74 years. The letters recommend a visit to the usual GP for a free FOBT kit and explain the screening programme and test procedures. Non-respondents receive a first reminder by mail explaining the need to visit their GP, and non-respondents to this reminder receive a second reminder by mail with the FOBT kit and a prepaid return envelope. Participants in the screening programme perform the FOBT at home then send the kit to the screening programme laboratory, which sends the results to the participants, usual GP, and local screening programme centre. The first two rounds of the population-based screening campaign occurred between June 2007 and December 2008 then between July 2009 and December 2010.
GPs should refer their patients with positive FOBT results to a gastroenterologist for colonoscopy. The colonoscopy results are sent to both the GP and the screening centre. When the screening centre receives no information about colonoscopy results, it mails reminders to the individual 3 and 6 months after the positive FOBT to request such results and to emphasise the need to see a gastroenterologist for colonoscopy; this second reminder specifies that no further FOBTs would be sent should the centre receive no information about colonoscopy; when data on the GP are available, the GP is also contacted. Individuals for whom no colonoscopy information is available 12 months after a positive FOBT are excluded from the programme.
Study population
All participants targeted by the programme and having positive FOBT results during either of the two screening rounds (2007–2008 or 2009–2010) were eligible for the present study. To leave sufficient time for the collection of colonoscopy data, we extracted the study data from the screening centre database on 20 February 2012.
Classification of individuals and outcomes
The study outcome was performance of colonoscopy after a positive FOBT. Individuals were classified according to performance and timing of colonoscopy. As no recommendations on colonoscopy timing were available, we defined early and delayed colonoscopy as performed before and after the median value, respectively; the median time-to-colonoscopy was 58 days (interquartile range, 40–98). Thus, individuals were classified into four mutually exclusive categories: early colonoscopy (⩽58 days), delayed colonoscopy (>58 days), no colonoscopy within 12 months, and no information on colonoscopy within 12 months.
The study outcome was first handled in two categories, namely, early colonoscopy or no early colonoscopy. Then, we considered all four above-described categories.
Potential predictors of colonoscopy
We considered potential predictors of colonoscopy related to the individual (level 1) and to the area of residence (level 2).
Individual predictors (level 1) extracted from the screening programme database included age, gender, and scheme of statutory health insurance coverage. In France, the statutory health insurance covers every resident. Different schemes coexist, depending on the person’s profession or situation (e.g., unemployment). We also assessed the following factors related to individual participation in the screening programme: screening FOBT performed for the first or second time and obtained in the first or second round, year of positive FOBT, availability of contact information for the usual GP, positive FOBT performed after the first letter or after the first or second reminder, FOBT kit received from the GP or by mail (second reminder), and time from FOBT receipt by the individual to completed FOBT receipt by the programme laboratory.
Contextual factors (level 2) were related to the individual’s census ward of residence (i.e., area) as determined based on the individual’s exact address. The census wards were those used by the National Institute of Statistics and Economic Studies. Contextual factors were socio-economic status, median GP density per 100 000 population, and local availability of a gastroenterologist. Aggregate socio-economic data were abstracted from the National Institute of Statistics and Economic Studies database and Maurice Halbwachs Centre database. We used the Townsend deprivation index (Townsend 1987), which measures material deprivation based on four census variables: unemployment, non-car ownership, non-home ownership, and household overcrowding. We obtained the values of three of the four variables from the 2008 National Institute of Statistics and Economic Studies census; the most recent available values for overcrowding were from the 1999 census. The Townsend deprivation index ranged from −8.58 to +11.13 and was categorised in quintiles with quintile 1 representing the least deprived and quintile 5 the most deprived areas.
Statistical analysis
Analyses were performed with STATA version 11.1 (StataCorp, College Station, TX, USA). Categorical variables are described as numbers and percentages and continuous variables as medians (interquartile range) or quintiles, as their distributions were generally not normal. To identify factors associated with absence of early colonoscopy, we first compared the characteristics of the early colonoscopy group with those of the other three groups pooled (delayed colonoscopy, no colonoscopy, and no information). We also separately assessed predictors of delayed colonoscopy, no colonoscopy, and no information, using early colonoscopy as the reference category. To investigate variability in colonoscopy uptake across GPs, we compared two groups, namely, early or delayed colonoscopy vs no colonoscopy or no information.
Multilevel models are particularly well suited to investigations of contextual effects, because they take into account the hierarchical structure of the data. We used a random-intercept logistic model with individuals (level 1) nested in areas (level 2). First, univariable analyses were performed by multilevel logistic regression. Crude odds ratios (ORs) with their 95% confidence intervals (95% CIs) were estimated. Variables with P values <0.15 by univariable analysis were selected for multivariable analysis. Confounders and interactions were tested in bivariate models. To avoid introducing highly correlated variables into the models (e.g., second postal reminder for FOBT and FOBT received by mail rather than from the GP), only the most relevant variables were entered into the multivariable models. To select the final models, we used the likelihood ratio test for nested models and the Bayesian Information Criterion for non-nested models.
We first used an empty model (including no variables; model 1) to investigate whether significant clustering occurred within areas for absence of early colonoscopy (Snijders and Bosker 1999). Then, we entered individual variables into the model (model 2). Third, level-2 variables were added to model 2 (models 3 and 4). Multilevel models can estimate the proportion of between-group variability related to contextual factors included in the model and can quantify the proportional change in variance (PCV) at the group level after the inclusion of level-1 and level-2 variables (Merlo et al, 2006). The PCV between two models was computed as follows: PCV=((VA−VB)/VA × 100), where VA was the variance of the initial model and VB the variance of the model containing additional variables. To compare differences in ORs for the delayed colonoscopy, no colonoscopy, and no information groups, we built a multinomial logistic regression model with robust variance estimates.
To investigate whether significant clustering occurred within GPs for absence of colonoscopy, we used an empty model with individuals at level 1 and GPs at level 2.
Sensitivity analysis
The robustness of delayed colonoscopy predictors was assessed using a linear multilevel model with time-to-colonoscopy (in days) as the dependent variable. The model was adjusted for the same variables as the multilevel logistic model.
All tests were two-sided, and P values ⩽0.05 were considered significant.
Results
Study population
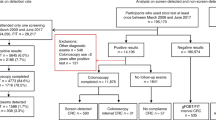
Among the 2369 individuals with positive FOBT results, 161 were excluded (Figure 1) and 2208 were analysed.
Table 1 reports the main study population characteristics. Median age was 61.5 years; 1987 individuals (90%) underwent colonoscopy, 998 (45.2%) within 58 days and 989 (44.8%) after 58 days; 102 (4.6%) did not undergo colonoscopy; and for 119 (5.4%) no information was available. Reasons for not undergoing colonoscopy were refusal by the individual (43.1%) or undergoing another FOBT instead of colonoscopy (25.5%); the reason was unknown for 31.4%. Colonoscopy was normal in 792 (39.9%) individuals and showed one or more adenomas in 942 (47.4%), CRC in 218 (11%) and cancer of another organ in 3 (0.1%). For 32 (1.6%) individuals known to have undergone colonoscopy, the colonoscopy results were unavailable.
Factors associated with delayed or no colonoscopy after positive FOBT
By univariable analysis (Table 2), absence of data on the usual GP, first screening FOBT, positive FOBT during the first screening round, FOBT received at home, and living in the most socio-economically deprived areas were significantly associated with not performing early colonoscopy. A trend towards an association (P<0.15) was found for FOBT after the second reminder and for residing in areas having high GP densities. Type of statutory health-care insurance was not significantly associated with early colonoscopy (P=0.41, data not shown).
The multilevel multivariable analysis showed significant variability across areas (P=0.01) (Table 3, empty model). Factors independently associated with not performing early colonoscopy were first screening FOBT (odds ratio; (OR), 1.64; 95% CI, 1.20–2.25), FOBT received at home (OR, 1.21; 95% CI, 0.99–1.49), and deprived (quintile 5) area (OR, 1.41; 95% CI, 1.06–1.89) (Table 3, final model). Area of residence with high GP density showed an independent association of borderline significance (OR, 1.17; 95% CI, 0.98–1.42). After adjustment for level-1 variables, differences across areas increased by only 1.3%, indicating that these differences were not explained by level-1 variables (model 1). Adjustment for Townsend index and GP density (model 3) decreased the variability across areas by 18.2%. Thus, socio-economic characteristics of the residence area accounted for about one-fifth of the disparity in colonoscopy uptake. By multinomial analysis (Table 4), compared with early colonoscopy, delayed colonoscopy was associated with first FOBT (OR, 1.61; 95% CI, 1.16–2.25) but not with socio-economic deprivation. Factors associated with no colonoscopy were similar to those associated with absence of information, and ORs in these two groups were similar (P of OR heterogeneity >0.28). Factors associated with no colonoscopy or no information were first FOBT (OR, 2.01; 95% CI, 1.02–3.97), FOBT received at home (OR, 2.29; 95% CI, 1.67–3.14), and socio-economically deprived areas (OR, 3.17; 95% CI, 1.98–5.08).
In the sensitivity analysis, longer time-to-colonoscopy was not associated with deprivation (P=0.28), FOBT receipt modality (P=0.52), or GP density (P=0.81), but was independently associated with first screening FOBT (P<0.05) (data not shown).
Variability across GPs
The empty multilevel model showed significant variation across GPs for colonoscopy uptake (P=0.01). By multivariable analysis, factors associated with no colonoscopy were FOBT received at home (OR, 2.37; 95% CI, 1.77–3.18) and living in a socio-economically deprived area (OR, 2.87; 95% CI, 1.80–4.34). First FOBT was not significantly associated with colonoscopy uptake. After adjustment, the level-2 variance remained significant (P=0.02), indicating that these variables did not explain the differences across GPs.
Discussion
No colonoscopy or absence of information on colonoscopy within 12 months after a positive FOBT was more common among individuals living in socio-economically deprived areas. Living in a deprived area was not associated with delayed colonoscopy. Colonoscopy uptake was higher when the FOBT was obtained from the GP than by mail at home, suggesting a positive role for GPs in patient adherence to colonoscopy requirements. No colonoscopy or delayed colonoscopy was more common after a FOBT performed for the first time as opposed to the second time. Significant variability across GPs for early or delayed colonoscopy uptake was found.
The high colonoscopy rate after a positive FOBT (90%) was consistent with previously reported rates (83.8–89.5%) (Steele et al, 2010; Dupont-Lucas et al, 2011; Morris et al, 2012; Moss et al, 2012). Median time-to-colonoscopy in our sample (58 days) was similar to that found in a French study (66 days) (Dupont-Lucas et al, 2011) and consistent with recommendations in the VHA directive, 2007 and Canadian consensus (Paterson et al, 2006).
Residence in a socio-economically deprived area was associated with no colonoscopy or no information about colonoscopy after a positive FOBT. During the English FOBT screening pilot, colonoscopy uptake was lowest in the most deprived areas but the association was not significant by multivariable analysis (Moss et al, 2012). A French study using aggregate socio-economic data showed no association with colonoscopy uptake after a positive FOBT (Dupont-Lucas et al, 2011). However, neither study used multilevel modelling. In keeping with our results, a recent study found that colonoscopy uptake varied with socio-economic deprivation of residence area, but these variations were small (Morris et al, 2012). People residing in socio-economically deprived areas may have limited access to information, difficulty understanding the screening process, or insufficient financial resources for colonoscopy. Although FOBT is provided free of charge, only 70% of colonoscopy costs are reimbursed by the French statutory health insurance system. Most French residents take out an optional supplemental health insurance policy to cover at least part of the remaining cost. The high fees practiced by private gastroenterologists in France may result in some patients having to pay for part of their colonoscopy costs out-of-pocket, which may constitute a strong disincentive to undergo colonoscopy. The association between socio-economic deprivation and colonoscopy uptake, but not with delayed colonoscopy, suggests that deprivation may affect colonoscopy uptake after a positive FOBT. Colonoscopy uptake was not significantly associated with local GP density, in keeping with a previous study on FOBT participation (Pornet et al, 2010), although a trend towards an association was observed for delayed colonoscopy. The ease of transportation in our district allows individuals to see GPs outside their area of residence. However, living in an area with many GPs but few gastroenterologists may result in long times to colonoscopy appointments. In keeping with our data, a French study found no association between colonoscopy uptake and access to gastroenterologists (Dupont-Lucas et al, 2011). High urbanisation in our district may explain that colonoscopy uptake was not associated with local gastroenterologist availability. Recent studies found small variations in colonoscopy uptake after a positive FOBT across age or gender subgroups (Steele et al, 2010; Morris et al, 2012), whereas others found no association with absence of colonoscopy, in keeping with our results (Etzioni et al, 2006; Rao et al, 2009; Dupont-Lucas et al, 2011; Moss et al, 2012).
The only factor associated with adherence to both FOBT and colonoscopy after a positive FOBT was the socio-economic status of the area of residence (Le Breton et al, 2012). We previously found a small degree of variability in FOBT uptake across GPs, who also had a significant influence on colonoscopy uptake in the present study. Some studies showed that GPs had a major influence on patient adherence to CRC screening procedures (Turner et al, 2003; Seeff et al, 2004; Turner et al, 2004; Rao et al, 2009), including colonoscopy after a positive FOBT. In a previous French study, rates of screening FOBT participation were 85% when the test was given by GPs and 15% when it was received at home (Viguier, 2009). Individuals in the no colonoscopy or no information groups may have failed to identify their GP as a resource for CRC screening. Thus, individuals who fail to communicate GP contact information to the screening programme centre may require close monitoring. The higher prevalence of delayed and absent colonoscopy after the first screening test may indicate greater concern generated by a positive test after a first negative test.
The colonoscopy rate varied across GPs. In a previous study, failure to perform a complete diagnostic evaluation after a positive FOBT was due to physician decisions in 33% of cases, and 43% of these physician decisions were inappropriate (Jimbo et al, 2009). In our study, 25.5% of individuals without colonoscopy underwent another FOBT, which may have been either requested by patients unwilling to undergo colonoscopy or prescribed by the GP.
Strengths and limitations
To our knowledge, this is the first study using multilevel modelling to simultaneously investigate individual and contextual factors associated with delayed or no colonoscopy after a positive FOBT.
Given the high quality of data collection by the local screening programme, most individuals in the no-information group probably failed to undergo colonoscopy. In addition, risk factors were similar in the no-colonoscopy and no-information groups. Consequently, we pooled these two groups. The Townsend index is an internationally recognised measure (Pornet et al, 2010; von Wagner et al, 2011) based on the assumption that, in the same area, most people share the same living conditions and socio-economic status. Using aggregate data avoids declarative bias in survey studies but does not allow conclusions about individuals (i.e., ecological bias) (Courgeau, 2004). Analyses of inequalities using aggregate data cannot separate individual effects from collective effects (Geronimus and Bound, 1998).
The Townsend index fails to take into account possible confounders such as marital or educational status. One of the index variables was available only from the 1999 census, which may have resulted in misclassification. GP and gastroenterologist densities are not accurate measures of health-care access (Guagliardo, 2004). We had limited information on GPs and were therefore unable to identify specific characteristics possibly associated with colonoscopy uptake by FOBT-positive patients.
Contextual socio-economic factors, first screening FOBT, and FOBT received at home instead of from the GP predicted failure to undergo colonoscopy after a positive FOBT. Delayed colonoscopy was more common after first than second screening FOBTs. Our results suggest that interventions to improve colonoscopy uptake after a positive FOBT should target populations in socio-economically deprived areas and individuals performing their first screening FOBT. Apart from mailed reminders to improve colonoscopy uptake, no other interventions have been implemented to date. Individual follow-up by the local screening programme centre should be strengthened, especially for individuals in deprived areas and those who do not receive the test from the GP. Moreover, practice heterogeneity exists across GPs, and interventions are needed to enhance the effectiveness of GPs in maximising colonoscopy uptake. GPs are in a unique position to provide appropriate messages tailored to individuals performing a first screening FOBT, with the goal of improving patient adherence to follow-up after a positive FOBT.
Change history
17 September 2013
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Chaix B, Chauvin P (2002) The contribution of multilevel models in contextual analysis in the field of social epidemiology: a review of literature. Rev Epidemiol Sante Publique 50: 489–499.
Courgeau D (2004) Du groupe à l’individu. Synthèse multiniveau. Institut national d’études démographiques: Paris, France.
Dupont-Lucas C, Dejardin O, Dancourt V, Launay L, Launoy G, Guittet L (2011) Socio-geographical determinants of colonoscopy uptake after faecal occult blood test. Dig Liver Dis 43: 714–720.
Etzioni D, Yano E, Rubenstein L, Lee ML, Ko CY, Brook RH, Parkerton PH, Asch SM (2006) Measuring the quality of colorectal cancer screening: the importance of follow-up. Dis Colon Rectum 49: 1002–1010.
Ferley J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM (2010) Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 127: 2893–2917.
Fisher DA, Jeffreys A, Coffman CJ, Fasanella K (2006) Barriers to full colon evaluation for a positive fecal occult blood test. Cancer Epidemiol Biomarkers Prev 15: 1232–1235.
Frederiksen BL, Jørgensen T, Brasso K, Holten I, Osler M (2010) Socioeconomic position and participation in colorectal cancer screening. Br J Cancer 103: 1496–1501.
Gellad Z, Almirall D, Provenzale D, Fischer DA (2009) Time from positive screening fecal occult blood test to colonoscopy and risk of neoplasia. Dig Dis Sci 54: 497–502.
Geronimus AT, Bound J (1998) Use of census-based aggregate variables to proxy for socioeconomic group: evidence from national samples. Am J Epidemiol 148: 475–486.
Guagliardo MF (2004) Spatial accessibility of primary care: concepts, methods and challenges. Int J Health Geogr 3: 3.
Hewitson P, Glasziou P, Watson E, Towler B, Irwig L (2008) Cochrane systematic review of colorectal cancer screening using the fecal occult blood test (Hemoccult): an update. Am J Gastroenterol 103: 1541–1549.
Jimbo M, Myers RE, Meyer B, Hyslop T, Cocroft J, Turner BJ, Weinberg DS (2009) Reasons patients with a positive fecal occult blood test result do not undergo complete diagnostic evaluation. Ann Fam Med 7: 11–16.
Le Breton J, Journy G, Attali C, Le Corvoisier P, Brixi Z, Bastuji-Garin S, Chevreul K (2012) Improving participation in colorectal cancer screening: targets for action. Prev Med 55: 488–492.
Merlo J, Chaix B, Ohlsson H, Beckman A, Johnell K, Hjerpe P, Råstam L, Larsen K (2006) A brief conceptual tutorial on multilevel analysis in social epidemiology: using measures of clustering in multilevel logistic regression to investigate contextual phenomena. J Epidemiol Community Health 60: 290–297.
Morris S, Baio G, Kendall E, von Wagner C, Wardle J, Atkin W, Halloran SP, Handley G, Logan RF, Obichere A, Rainbow S, Smith S, Snowball J, Raine R (2012) Socioeconomic variation in uptake of colonoscopy following a positive faecal occult blood test result: a retrospective analysis of the NHS Bowel Cancer Screening Programme. Br J Cancer 107: 765–771.
Moss SM, Campbell C, Melia J, Coleman D, Smith S, Parker R, Ramsell P, Patnick J, Weller DP (2012) Performance measures in three rounds of the English bowel cancer screening pilot. Gut 61: 101–107.
Paterson WG, Depew WT, Paré P, Petrunia D, Switzer C, Veldhuyzen van Zanten SJ, Daniels S Canadian Association of Gastroenterology Wait Time Consensus Group (2006) Canadian consensus on medically acceptable wait times for digestive health care. Can J Gastroenterol 20: 411–423.
Pornet C, Dejardin O, Morlais F, Bouvier V, Launoy G (2010) Socioeconomic determinants for compliance to colorectal cancer screening. A multilevel analysis. J Epidemiol Community Health 64: 318–324.
Rao S, Schilling T, Sequist T (2009) Challenges in the management of positive fecal occult blood tests. J Gen Intern Med 24: 356–360.
Seeff LC, Nadel MR, Klabunde CN, Thompson T, Shapiro JA, Vernon SW, Coates RJ (2004) Patterns and predictors of colorectal cancer test use in the adult US population. Cancer 100: 2093–2103.
Snijders TAB, Bosker RJ (1999) Multilevel analysis: an introduction to basic and advanced multilevel modeling. Sage Publications Ltd: London, UK.
Steele RJ, Kostourou I, McClements P, Watling C, Libby G, Weller D, Brewster DH, Black R, Carey FA, Fraser C (2010) Effect of gender, age and deprivation on key performance indicators in a FOBT-based colorectal screening programme. J Med Screen 17 (2): 68–74.
Townsend P (1987) Deprivation. J Soc Pol 16: 125–146.
Turner B, Myers RE, Hyslop T, Hauck WW, Weinberg D, Brigham T, Grana J, Rothermel T, Schlackman N (2003) Physician and patient factors associated with ordering a colon evaluation after a positive fecal occult blood test. J Gen Intern Med 18: 357–363.
Turner BJ, Weiner M, Yang C, TenHave T (2004) Predicting adherence to colonoscopy or flexible sigmoidoscopy on the basis of physician appointment-keeping behavior. Ann Intern Med 140: 528–532.
US Department of Veterans Affairs (2007) VHA directive 2007–004 Colorectal Cancer Screening http://www1.va.gov/vhapublications/ViewPublication.asp?pub_ID=1530 (last accessed on 21 February 2013).
Viguier J (2009) L’organisation du dépistage du cancer colorectal en France. Bull Epidemiol Hebd 2-3: 19–22.
von Wagner C, Baio G, Raine R, Snowball J, Morris S, Atkin W, Obichere A, Handley G, Logan RF, Rainbow S, Smith S, Halloran S, Wardle J (2011) Inequalities in participation in an organized national colorectal cancer screening programme: results from the first 2.6 million invitations in England. Int J Epidemiol 40: 712–718.
Weller D, Coleman D, Robertson R, Butler P, Melia J, Campbell C, Parker R, Patnick J, Moss S (2007) The UK colorectal cancer screening pilot: results of the second round of screening in England. Br J Cancer 97: 1601–1605.
Acknowledgements
We thank Iradj Sobhani, MD, PhD; Florence Canouï-Poitrine, MD, PhD; Etienne Audureau, MD, PhD; and Isabelle Durand-Zaleski, MD, PHD, for contributing to the study discussion; and A. Wolfe, MD, for revising the manuscript. The study was approved by the ‘Comité de protection des Personnes Ile-de-France IX.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
This work was presented in part at the ESMO congress, October 2012, Vienna, Austria.
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Ferrat, E., Le Breton, J., Veerabudun, K. et al. Colorectal cancer screening: factors associated with colonoscopy after a positive faecal occult blood test. Br J Cancer 109, 1437–1444 (2013). https://doi.org/10.1038/bjc.2013.476
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bjc.2013.476
Keywords
This article is cited by
-
Implementing a multilevel intervention to accelerate colorectal cancer screening and follow-up in federally qualified health centers using a stepped wedge design: a study protocol
Implementation Science (2020)
-
The Need for an Integrated Patient Navigation Pathway to Improve Access to Colonoscopy After Positive Fecal Immunochemical Testing: A Safety-Net Hospital Experience
Journal of Community Health (2017)
-
Timeliness of Colonoscopy After Abnormal Fecal Test Results in a Safety Net Practice
Journal of Community Health (2016)
-
Nationwide bowel cancer screening programme in England: cohort study of lifestyle factors affecting participation and outcomes in women
British Journal of Cancer (2015)
-
Predictors of adherence to repeat fecal occult blood test in a population-based colorectal cancer screening program
British Journal of Cancer (2014)