Abstract
Introduction
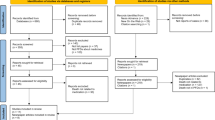
Coroners inquire into sudden, unexpected, or unnatural deaths. We have previously established 99 cases (100 deaths) in England and Wales in which medicines or part of the medication process or both were mentioned in coroners’ ‘Regulation 28 Reports to Prevent Future Deaths’ (coroners’ reports).
Objective
We wished to see what responses were made by National Health Service (NHS) organizations and others to these 99 coroners’ reports.
Methods
Where possible, we identified the party or parties to whom these reports were addressed (names were occasionally redacted). We then sought responses, either from the UK judiciary website or by making requests to the addressee directly or, for NHS and government entities, under the Freedom of Information Act 2000. Responses were analysed by theme to indicate the steps taken to prevent future deaths.
Results
We were able to analyse one or more responses to 69/99 cases from 106 organizations. We analysed 201 separate actions proposed or taken to address the 160 concerns expressed by coroners. Staff education or training was the most common form of action taken (44/201). Some organisations made changes in process (24/201) or policy (17/201), and some felt existing policies were sufficient to address some concerns (22/201).
Conclusions
Coroners’ concerns are often of national importance but are not currently shared nationally. Only a minority of responses to coroners’ reports concerning medicines are in the public domain. Processes for auditing responses and assessing their effectiveness are opaque. Few of the responses appear to provide robust and generally applicable ways to prevent future deaths.
Similar content being viewed by others
References
Pirmohamed M, James S, Meakin S, Green C, Scott AK, Walley TJ, et al. Adverse drug reactions as cause of admission to hospital: prospective analysis of 18 820 patients. BMJ. 2004;329:15–9.
Montané E, Arellano AL, Sanz Y, Roca J, Farré M. Drug-related deaths in hospital inpatients: a retrospective cohort study. Br J Clin Pharmacol. 2018 Mar;84(3):542–52.
Pardo Cabello AJ, Del Pozo Gavilán E, Gómez Jiménez FJ, Mota Rodríguez C, de Luna Del Castillo J, Puche Cañas E. Drug-related mortality among inpatients: a retrospective observational study. Eur J Clin Pharmacol. 2016;72(6):731–6.
United Kingdom Courts and Tribunals Judiciary. https://www.judiciary.uk/related-offices-and-bodies/office-chief-coroner/pfd-reports/. Accessed 29 Aug 2018.
Ferner RE, Easton C, Cox AR. Deaths from medicines: a systematic analysis of coroners’ reports to prevent future deaths. Drug Saf. 2018;41(1):103–10.
Anonymous. Pubmed Time-line. https://www.ncbi.nlm.nih.gov/pubmed/?term=medication+error. Accessed 03 Sep 2018.
Donaldson L. An organisation with a memory. Clin Med (Lond). 2002;2(5):452–7.
Anonymous. What is the Freedom of Information Act? Information Commissioner’s Office. https://ico.org.uk/for-organisations/guide-to-freedom-of-information/what-is-the-foi-act/. Accessed 10 July 2018.
Gandhi TK, Berwick DM, Shojania KG. Patient safety at the crossroads. JAMA. 2016;315(17):1829–30.
Berwick DM, Enthoven A, Bunker JP. Quality management in the NHS: the doctor’s role. BMJ. 1992;304(6821):235–9.
Hunt J. NHS: learning from mistakes, 9th March 2016. Hansard 2016;607(Column 295). http://bit.ly/2NBxFOq. Accessed 3 Sep 2018.
Sutherland G, Kemp C, Studdert DM. Mandatory responses to public health and safety recommendations issued by coroners. Aust NZ J Public Health. 2016;40:451–60.
Gosport Independent Panel. The inquests, chapter 8. In: Gosport War Memorial Hospital. The Report of the Gosport Independent Panel. https://www.gosportpanel.independent.gov.uk. Accessed 8 July 2018.
Coroners Court of Victoria. State government of Victoria. Death investigation process; 2017. http://www.coronerscourt.vic.gov.au/resources/1e321087-60c8-4c56-beb3-63d29d263c81/coronial+processes.pdf. Accessed 29 Aug 2018
Introduction to Recommendations recap. A summary of coronial recommendations and comments made between 1 July 2017 and 31 December 2017. https://coronialservices.justice.govt.nz/assets/Documents/Publications/issue-14-recommendations-recap2.pdf. Accessed 29 Aug 2018.
Coronial Services of New Zealand. Findings and recommendations. https://coronialservices.justice.govt.nz/findings-and-recommendations/. Accessed 29 Aug 2018.
Moore J. Coroners’ recommendations about healthcare-related deaths as a potential tool for improving patient safety and quality of care. New Zealand Med J 2014;127 (1398). http://www.nzma.org.nz/journal/read-the-journal/all-issues/2010-2019/2014/vol-127-no.-1398/6212. Accessed 03 Sep 2018.
Jokanovic N, Ferrah N, Lovell JJ, et al. A review of coronial investigations into medication-related deaths in Australian residential aged care. Res Soc Admin Pharm. 2018. https://doi.org/10.1016/j.sapharm.2018.06.007.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The West Midlands Centre for Adverse Drug Reactions receives funding from the Medicines and Healthcare products Regulatory Agency (MHRA).
Conflict of interest
Robin Ferner has provided medicolegal reports for coroners and others. Tohfa Ahmad, Zainab Babatunde and Anthony R. Cox have no conflicts of interest directly relevant to the content of this study.
Ethical approval
This study was an analysis of publicly available data. No approval was sought.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ferner, R.E., Ahmad, T., Babatunde, Z. et al. Preventing Future Deaths from Medicines: Responses to Coroners’ Concerns in England and Wales. Drug Saf 42, 445–451 (2019). https://doi.org/10.1007/s40264-018-0738-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40264-018-0738-z