Abstract
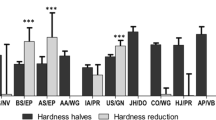
The aim of this study was to evaluate the weight uniformity of commonly divided tablets produced by Palestinian Pharmaceutical Companies and to evaluate the importance of both patient- and formulation-related variables on the splitting results. Eighty-four volunteers were enrolled in this study; their age, gender and occupation were documented in order, and the effect of these variables on the tablet splitting results was evaluated. Each volunteer was asked to divide six scored tablets of each product tested and was given clear instructions on how to conduct the splitting process. The split units were individually weighed and the RSD for each product was calculated as instructed in the European Pharmacopoeia (Ph. Eur. 5.5). Only one scored tablet product passed the Ph. Eur. test of mass uniformity, while the remaining 13 products failed; this indicates that the splitting of these tablet products is not a reliable means for the provision of accurate doses to patients. Age, gender and occupation of volunteers were not found to be predictive of any variability noted in the splitting results. The only factors that were suspected to be linked to passing the splitting test, as per the European Pharmacopoeia, were the shape, friability and hardness of the tablets. As a result of this study, we believe that the practice of dividing tablets, which should provide therapeutic and economic benefits for the patient, may potentially cause significant problems, especially in drugs with low therapeutic indices. Tablets produced by Palestinian Pharmaceutical Companies should comply with the new Ph. Eur. splitting regulations to reduce this potential for complications.
Similar content being viewed by others
References
Anonymous, Note for Guidance on Development Pharmaceutics. Web-site EMEA: http://www.emea.eu.int. (1998).
APhA Strategic Directions Committee. Tablet splitting: evaluating appropriateness for patients. J. Am. Pharm. Assoc., 44,3, 324–325 (2004).
Biron, C., Licznar, P., Hansel, S., and Schved, J. F., Oral anticoagulant drugs: do not cut tablets in quarters. Thromb. Haemost., 82, 1201 (1999).
Carr-Lopez, S. M., Mallet, M. S., and Morse, T., The tablet splitter: barrier to compliance or cost-saving instrument? Am. J. Health Syst. Pharm., 52, 2707–2708 (1995).
Duncan, M. C., Castle, S. S., and Streetman, D. S., Effect of tablet splitting on serum cholesterol concentrations. Ann. Pharmacother., 36, 205–209 (2002).
Fawell, N. G., Cookson, T. L., and Scranton, S. S., Relationship between tablet splitting and compliance, drug acquisition cost, and patient acceptance. Am. J. Health Syst. Pharm., 56, 2542–2545 (1999).
Gupta, P. and Gupta, K., Broken tablets: does the sum of the parts equal the whole? Am. J. Hosp. Pharm., 45, 1498 (1988).
Kristensen, H. G., Jorgensen, G. H., and Sonnergaard, J. M., Mass uniformity of tablets broken by hand. Pharmeuropa, 7, 298–302 (1995).
Lüdemann, J. and Moest, T., Dosierungsgenauigkeit teilbarer tabletten. Realisierung uber die tablettenform. Dtsch. Apoth. Ztg., 134, 27–30 (1994).
Marriott, J. L. and Nation, R. L., Splitting tablets. Australian Prescriber, 25, 133–135 (2002).
McDevitt, J. T., Gurst, A. H., and Chen, Y., Accuracy of tablet splitting. Pharmacotherapy, 18, 193–197 (1998).
Rodenhuis, N., de Smet P. A. G. M., and Barends, D. M., Patient experiences with the performance of tablet score lines needed for dosing. Pharm. World Sci., 25, 173–176 (2003).
Rosenberg, J. M., Nathan, J. P., and Plakogiannis, F., Weight variability of pharmacist-dispensed split tablets. J. Am. Pharm. Assoc., 42, 200–205 (2002).
Schumann, C., Neue Tablettenform: Exakt Teilbar New tablet form: accurate divisible. Pharm. Ztg., 140, 39–45 (1995).
Spang, R., Breakability of tablets and film-coated dragees. Pharm. Acta Helv., 57, 99–111 (1982).
Tablets, European Pharmacopoeia Suppl. 4.1, Council of Europe; European Directorate for the Quality of Medicine, Strasbourg, Monograph 0478 (2002).
van Santen, E., Barends, D. M., and Frijlink, H. W., Breaking of scored tablets: a review. Eur. J. Pharm. Biopharm., 53, 139–145 (2002).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zaid, A.N., Ghosh, A.A. Compliance of scored tablet halves produced by Palestinian Pharmaceutical Companies with the new European Pharmacopoeia requirements. Arch. Pharm. Res. 34, 1183–1189 (2011). https://doi.org/10.1007/s12272-011-0717-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12272-011-0717-8