Abstract
Purpose
Pain, fatigue and depression are common sequelae of a cancer diagnosis. The extent to which these occur together in prostate cancer survivors is unknown. We (i) investigated prevalence of the pain-fatigue-depression symptom cluster and (ii) identified factors associated with experiencing the symptom cluster among prostate cancer survivors.
Methods
Men in Ireland diagnosed with prostate cancer 2–18 years previously were identified from population-based cancer registries and sent postal questionnaires. Cancer-related pain and fatigue were measured using the EORTC QLQ-C30 and depression using the DASS-21. Cut-offs to define ‘caseness’ were pain ≥ 25, fatigue ≥ 39 and depression ≥ 10. Associations between survivor-related factors, clinical variables and specific prostate cancer physical symptoms and the symptom cluster were assessed using multivariate logistic regression.
Results
A total of 3348 men participated (response rate = 54%). Twenty-four percent had clinically significant pain, 19.7% had clinically significant fatigue, and 14.4% had depression; 7.3% had all three symptoms. In multivariate analysis, factors significantly associated with the symptom cluster were living in Northern Ireland, experiencing back pain at diagnosis and being affected by incontinence, loss of sexual desire, bowel problems, gynecomastia and hot flashes post-treatment. There was a strong association between the cluster and health-related quality of life.
Conclusions
The pain-fatigue-depression symptom cluster is present in 1 in 13 prostate cancer survivors. Physical after-effects of prostate cancer treatment are associated with this cluster. More attention should be paid to identifying and supporting survivors who experience multiple symptoms; this may help health-related quality of life improve among the growing population of prostate cancer survivors.
Similar content being viewed by others
Introduction
Among men, prostate cancer is the second most common cancer, after lung cancer, with an estimated 1.28 million new cases diagnosed worldwide in 2018 [1]. Over the past decade, prostate cancer incidence rates have risen substantially, especially in Northern and Western Europe [2]. Survival rates for prostate cancer are high (and rising) so that more men are living with this cancer than any other form of cancer [3, 4].
A range of treatment options are available for prostate cancer, including radical prostatectomy (RP), radiotherapy (external beam (EBRT) or brachytherapy (BT)), observation (active surveillance (AS) or watchful waiting (WW)) and chemotherapy. However, none of these are clearly associated with lower mortality, at least for localized disease [5]. In addition, all of the treatments pose a high risk of adverse physical effects (e.g. incontinence of urine, bowel problems and erectile dysfunction) as well as the more generalized cancer-related symptoms (e.g. pain, insomnia, fatigue), all of which can persist long-term [6, 7]. Moreover, survivors have poorer psychological wellbeing than men in the general population [8].
Cancer-related pain, fatigue and depression are recognized to co-exist in a ‘symptom cluster’ among cancer patients and survivors [9,10,11]. Moreover, there is some evidence that the immune/inflammation pathway provides a biological basis for the co-existence of these symptoms in a cluster [12]. However, a 2017 Expert Panel observed that symptom cluster research remains extremely limited but that the increasing focus on personalized care means that it is crucial that an understanding of individual susceptibility to symptoms and clusters of these is better understood [13].
The individual elements of the symptom cluster are common among prostate cancer survivors. Up to three-quarters of survivors may experience cancer-related fatigue; urethral pain is reported by 16% following radiation therapy; and on average, 18% of survivors have depression post-treatment [14,15,16]. Moreover, associations have been reported between pairs of elements of the cluster (e.g. depression and fatigue; pain and mental health) in prostate cancer survivors [8, 17]. However, as far as we are aware, no studies have investigated prevalence of the pain-fatigue-depression symptom cluster in prostate cancer or which survivors are at greatest risk of experiencing the cluster. Such information could be valuable in informing targeting of supportive care interventions.
The aim of this analysis was to (i) investigate prevalence of the pain-fatigue-depression symptom cluster among prostate cancer survivors and (ii) identify factors associated with experiencing the symptom cluster.
Methods
Subjects
The study setting was the island of Ireland, which comprises the Republic of Ireland (RoI) and Northern Ireland (NI; part of the UK). High-quality population-based cancer registries exist in both NI and RoI. Study methods have been described in detail elsewhere [18]. Briefly, all men diagnosed with invasive prostate cancer (ICD10 C61) between 1st January 1995 and 31st March 2010 and who were still alive on 31st March 2011 were identified through the cancer registries. A stratified random sample (n = 12,322, 54% of sampling frame) was selected, to ensure approximately equal numbers < 5 and ≥ 5 years post-diagnosis in both jurisdictions, and screened for eligibility by healthcare providers (HCPs), GPs in RoI and hospital nurses in NI. To be eligible, men had to be (1) alive, (2) aware of their prostate cancer diagnosis, (3) well enough to receive and complete a questionnaire (in particular, have no cognitive impairment), (4) able to understand English and (5) usually reside in RoI or NI. Following screening, 6559 survivors were considered eligible.
Data collection
Eligible survivors were invited to complete a postal questionnaire, a copy of which is available from the authors on request. Non-responders were sent two reminders at two weekly intervals. The questionnaire collected information on sociodemographic characteristics; health at diagnosis, including presence of urinary symptoms (increased frequency, pain during urination, blood in urine), sexual symptoms (erectile dysfunction/impotence) or comorbidities (lung or heart disease, stroke, depression, diabetes, high blood pressure, bowel problems (constipation/diarrhoea), diverticular disease); mode of diagnosis (asymptomatic/prostate-specific antigen (PSA) detected, symptomatic/clinically detected, other); and treatment(s) received (RP, EBRT, androgen deprivation therapy (ADT), BT, chemotherapy, AS/WW). Men were asked to identify whether they had ever or currently experienced any of six physical after-effects following treatment (incontinence, impotence, loss of sexual desire, bowel problems (diarrhoea/constipation), gynecomastia, hot flashes/flushes or sweats). These after-effect questions, the ones present on symptoms at diagnosis, and mode of detection were developed by the authors [19, 20]. Cancer-related pain and fatigue were assessed using the European Organization for Research and Treatment of Cancer Quality-of-Life Questionnaire Version 3.0 (EORTC QLQ-C30) [21]. The global health score questions on this instrument provided a measure of health-related quality of life (HRQoL). Depression was assessed using the 21-question version of the Depression, Anxiety and Stress Scale (DASS-21) [22]. The questionnaire was pretested for face validity, acceptability and ease of completion among men with prostate cancer prior to being used. Information on clinical stage and Gleason grade at diagnosis and time since diagnosis was abstracted from cancer registry records.
The study was approved by the Ethics Committee of the Irish College of General Practitioners in the RoI and the NI Office for Research Ethics. Participants provided written informed consent.
Statistical analysis
Of the 6559 survivors sent questionnaires, 297 were subsequently discovered to have died [18]; 3348 returned a completed questionnaire and were included in the analysis dataset. The three outcomes of interest – fatigue, pain and depression – were scored as recommended [23, 24]. For fatigue and pain, we used pro-rating to impute missing responses for subjects who answered at least half, but not all, questions in the relevant subscale; in these instances, missing responses were filled with the mean value for that subject’s responses to the questions they did answer; a fatigue score was imputed for 138 respondents (4.1%) and a pain score for 126 respondents (3.8%). The range of possible scores for fatigue and pain was 0–100 and for depression was 0–42. Initially the three variables were summarized, and Pearson correlations were computed for each pair of variables. Then, three binary variables were created classifying men according to whether or not they scored in the range for clinically significant cancer-related pain, clinically significant cancer-related fatigue and depression. The cut-offs used to define ‘caseness’ were ≥ 25 on the pain scale; ≥ 39 on the fatigue scale; and ≥ 10 on the depression scale [22, 24]. An outcome symptom cluster variable was constructed based on the presence of pain, fatigue and depression, with categories none, any one, any two and all of three symptoms. Chi-square tests were used to compare survivor characteristics by symptom cluster categories.
A multivariable logistic regression model of factors associated with presence of the symptom cluster was developed using a forward-stepwise selection approach (using a significant level < 0.05 for inclusion). In this analysis, the symptom cluster variable was collapsed into two categories: < 3 symptoms (none/any one/any two symptoms) vs all three symptoms. The candidate variables for inclusion in all models were sociodemographic characteristics (age at diagnosis and survey, country of residence, marital status, whether lived alone at diagnosis, highest level of education completed, working status, first-degree family history of prostate cancer); diagnosis characteristics (urinary or sexual symptoms at diagnosis, mode of diagnosis, comorbidities, time since diagnosis, Gleason score at diagnosis, clinical stage at diagnosis); treatment(s) received; current physical after-effects; and overall HRQoL (classified as ≥ or < median score) (Table 1). Since complete case analyses are usually biased, for the covariates, if more than 3% of men had missing data, ‘missing’ was included as a category. Goodness of fit of the final models was checked using the Hosmer and Lemeshow test. A two-sided p value of < 0.05 was considered to be statistically significant throughout. Analyses were performed using STATA V.15.0.
Results
Of the 3348 men who responded to the questionnaire, slightly more than a quarter were 70 years of age or older at diagnosis; 35% had completed only primary level education; and almost a quarter had a first-degree family history of prostate cancer (Table 1). Presence of symptoms pre-diagnosis ranged from 7% for blood in urine to 51% for frequent urination. Nearly half of participants were 2–5 years since diagnosis. Almost one quarter had advanced stage (stage 3/4) disease at diagnosis. The most common treatments received were EBRT (51%) and RP (28%). The most common current treatment after-effects were loss of sexual desire (47%) and impotence (59%). The median HRQoL score was 75.0 (out of a possible 100).
Prevalence of pain, fatigue and depression
Overall, at the time of the survey, 660 men (19.7%) reported clinically significant cancer-related fatigue, 802 (24.0%) had clinically significant cancer-related pain, and 481 (14.4%) had depression.
Mean scores for the three symptoms were fatigue 24.0 (sd = 24.29, lowest = 0, highest = 100); pain 15.4 (sd = 25.0, lowest = 0, highest = 100); and depression 4.5 (sd = 7.71, lowest = 0, highest = 42). Men’s scores for each pair of symptoms were strongly, and statistically significantly, correlated (pain and fatigue, rho = 0.650; depression and fatigue, rho = 0.564; depression and pain, rho = 0.454; all p < 0.001).
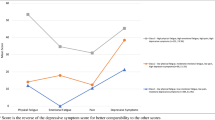
A total of 2879 (86.0%) men completed the pain, fatigue and depression scales and were therefore included in the symptom cluster analysis. Figure 1 demonstrates combinations of pain, fatigue and depression. A total of 1024 (35.6%) men experienced one or more of the symptoms in the cluster. A total of 127 men (4.4%) were affected solely by fatigue, 260 (9.0%) were affected by pain alone, and 150 (5.2%) were affected by only depression. A total of 278 men (9.7%) experienced two symptoms: fatigue and pain, 161 (5.6%); fatigue and depression, 59 (2.1%); and pain and depression, 58 (2.0%). Two hundred nine men (7.3%) had all three symptoms. Of the men who reported at least one symptom, almost half experienced two or more (two symptoms, 27.2%; all three symptoms, 20.4%).
Prevalence of fatigue, pain and depression and combinations of these in prostate cancer survivors, expressed as (a) number (percentage) of survivors who completed the fatigue, pain or depression scales (n = 2879) and (b) number (percentage) of survivors who experienced at least one of the symptoms (n = 1024)
Factors associated with the symptom cluster
Table 2 displays the study characteristics by the four symptom cluster categories (no symptoms, one symptom, two symptoms and all three symptoms). There were significant differences in age at diagnosis, country of residence, marital status, living alone at diagnosis, highest level of education, employment status at diagnosis, mode of diagnosis, symptoms pre-diagnosis and post-treatment, clinical stage, Gleason grade, treatment received and HRQoL across the symptom cluster categories (all p < 0.05). Men in Northern Ireland, who lived alone, had only primary level education and, who were retired, more frequently had the pain-fatigue-depression symptom cluster. The symptom cluster was associated with having clinically detected cancer and symptoms, comorbidities, higher stage and higher Gleason grade at prostate cancer diagnosis. It was also associated with each of the six post-treatment physical symptoms assessed (urinary incontinence, impotence, loss of sexual desire, gynecomastia, sweats/hot flashes).
Table 3 shows the variables that were significantly associated with presence of the symptom cluster (i.e. experiencing all three symptoms) in the multivariable model. Risk of experiencing the symptom cluster was nearly 3-fold higher in men in NI than RoI and two-fold higher in those with primary only compared to tertiary education (OR = 2.01, 95%CI 1.29–3.12). Two pre-diagnosis symptoms were significantly associated with the symptom cluster: urinating more frequently (OR = 1.53, 95% CI 1.05–2.23) and back pain (OR = 2.22, 95%CI 1.51–3.27). Four post-treatment physical symptoms were significantly related to the symptom cluster: incontinence of urine (OR = 1.91, 1.33–2.76); bowel problems (OR = 1.95, 95%CI 1.36–2.80); gynecomastia (OR = 2.06, 95%CI 1.35–3.14); and sweats or hot flashes (OR = 1.56, 95%CI 1.07–2.26). Lower HRQoL was very strongly related to the symptom cluster (OR = 44.09, 95%CI 17.88–108.73).
Discussion
This study investigated the prevalence and factors associated with the pain-fatigue-depression symptom cluster among prostate cancer survivors. Several sociodemographic and prostate cancer specific symptoms were notably associated with this symptom cluster in univariate analyses. Once these symptoms were included in the multivariable model, many of the previously statistically significant sociodemographic and clinical factors became non-significant. The variables that remained in the model largely related to physical symptoms present pre-diagnosis (frequent urination and back pain) and after treatment (incontinence, bowel problems, gynecomastia, hot flashes). This suggests that cancer-related symptoms are more important indicators of being likely to experience this symptom cluster than sociodemographic and (majority of) clinical factors. The study also demonstrates that the three elements of the system cluster can occur alone, in pairs or all together in prostate cancer survivors, confirming previous, more general, observations [25].
Prevalence of the symptom cluster
There is emerging (albeit inconsistent) data which suggest that inflammatory and neuroimmune markers, such as cytokines, may explain the clustering of pain, fatigue and depression in people with cancer [12]. As far as we are aware, this is the first study of this specific symptom cluster in prostate cancer survivors, and 7.3% (approximately one in every 13 survivors) experienced all three symptoms. Comparative prevalence estimates are available from relatively few studies, in part because authors have investigated and reported a variety of different combinations of symptoms as potential clusters [26]. A further complication is that, even among studies that have examined pain, fatigue and depression, different instruments were used to assess these. A recent study of 606 gastrointestinal cancer patients reported that 9.6% experienced the fatigue-pain-depression symptom cluster [27]. Higher figures have been reported in studies of patients with lung cancer (19%), which is often advanced at diagnosis, patients with advanced cancer (20%) and patients following a palliative pathway (27%) [11, 28, 29]. In terms of possible explanations for the lower observed prevalence of the symptom cluster in the current study population, this may be because the majority of survivors had localized disease at diagnosis and many had survived 10 years or more. In addition, symptoms had to be scored at a level considered clinically important to be counted.
There are many more men living with prostate cancer worldwide than with any other cancer (3.7 million men within 5 years of diagnosis alone) [30]. This indicates that, despite the lower prevalence of the symptom cluster in prostate cancer than in (some) other cancer populations, very large numbers of prostate cancer survivors worldwide may be experiencing the combination of pain, fatigue and depression (with many more living with one or two of these symptoms).
Factors associated with the symptom cluster
As noted above, studies in which the participants had advanced cancer have generally reported higher prevalence of the symptom cluster than the current study. The observed associations between presence of physical symptoms pre-diagnosis (frequent urination and back pain) and the symptom cluster may be because these symptoms can indicate more advanced disease at diagnosis [31, 32]; thus, physical symptoms here may simply be acting as a marker of more advanced cancer. Similarly, presence of comorbid conditions has been linked with higher stage at prostate cancer diagnosis [33].
The study population was diagnosed over a long period, and there were changes in prostate cancer treatment over that time; notably radiotherapy became much more widely used, and brachytherapy was introduced [34]. We have previously reported variation in the prevalence of post-treatment symptoms among prostate cancer survivors according to primary treatment(s) received [19]. Over the time of the study, there were also differences between RoI and NI in the frequency with which different treatments were used [35]. This may help to explain the observed association between country of residence and presence of the symptom cluster in multivariable analyses.
Gynecomastia and hot flashes – two of the four post-treatment symptoms related to the symptom cluster – are side-effects of androgen deprivation therapy. Studies have previously indicated that these are associated with stigma, shame, loss of masculinity and psychological distress [36, 37]. As regards urinary incontinence, men who experience this may fear smelling or leakage of urine and find using incontinence pads embarrassing [38]; this may lead to social isolation and increased risk of depression [39]. Although bowel dysfunction has been noted to be particularly aggravating for prostate cancer survivors [40], men’s experiences of this, and its impact, have not been well investigated. It is possible that men experience discomfort due to diarrhoea or constipation and, as for urinary incontinence, may worry about leakage and embarrassment from wearing bowel incontinence pads. As well as the adverse psychological and physical effects, these physical symptoms may cause sleep disturbance [41]; sleep disorders, in turn, are linked with cancer-related fatigue [42]. Thus, the constellation of consequences of these post-treatment symptoms may explain their association with the pain-fatigue-depression symptom cluster.
Laird et al. [28] reported a strong association between the symptom cluster and worse physical functioning. We have extended these findings by showing a very strong association between lower HRQoL and the symptom cluster, which was evident after adjustment for post-treatment physical symptoms which, themselves, might be expected to impact on physical functioning. This finding has important implications.
Implications
The current findings suggest that intervention to alleviate elements of the symptom cluster might improve survivors’ HRQoL. Both pharmacological and non-pharmacological treatments and/or interventions have been shown to be effective for the components of the cluster. Pain can be treated using resistance exercise techniques and medications [43, 44]. Mood disorders, including depression, can be treated using medication and a variety of psychotherapies, depending on the cause [45]. Physical activity also improves depression and HRQoL among survivors [46]. Cognitive-behavioural therapies, pharmacological agents and, again, physical activity (aerobic or resistance) can be effective for treating cancer-related fatigue [47, 48]. Considering this, it is possible that an intervention/treatment that is competent in addressing one symptom of the cluster (e.g. physical activity) may also alleviate another symptom (or, indeed, improve HRQoL). This provides, as noted previously by Fleishman [25], an opportunity to be imaginative in planning of survivor care and treatment strategies. However, data from the current study population indicates that supportive interventions to alleviate symptoms of prostate cancer treatment are currently not frequently used [49]. Moreover, research among clinicians indicates considerable uncertainty in how to manage concurrent symptoms in cancer patients and survivors [50], suggesting professional education initiatives may be needed.
Strengths and limitations
Significant strengths of this study include the use of population-based sampling frames to identify survivors and the large sample size. As with any survey, there may be systematic differences between responders and non-responders. The 3348 survey respondents were younger and more often from RoI than non-respondents [18]. Among respondents, the 2879 men who answered all three sets of symptom questions were older, more often from NI, and had higher educational levels, making it unclear whether prevalence of the symptom cluster is likely to have been under- or overestimated. Measurement of the components of the symptom cluster is not straightforward. While we used validated questionnaires [21, 22] and thresholds previously shown to be associated with clinical importance [24], the EORTC QLQC30 includes only three items on fatigue and two on pain; the use of other instruments focussed specifically on cancer-related fatigue or pain, such as the EORTC QLQ-FA12 [51], may have provided richer information. We developed the questions on physical symptoms pre-diagnosis and post-treatment symptoms ourselves, and while these were pre-tested among prostate cancer survivors, information is lacking on their validity and psychometric properties; this is a limitation. The cross-sectional design means care must be taken in interpretation, particularly with regard to the direction of associations between the symptom cluster and post-treatment symptoms and HRQoL. It is possible that men with depression, fatigue and pain may assess their urinary incontinence or gynecomastia (e.g.) as worse than men who are not experiencing the symptom cluster. We have been unable to identify any population-level data on the prevalence of the combination of fatigue, pain and depression so we cannot comment on whether this is more frequent among prostate cancer survivors than the general male population.
Finally, while our focus was on the specific combination of pain, fatigue and depression among prostate cancer survivors, clusters of different combinations of symptoms among survivors of other cancers have been reported [see, e.g. 51,52,53,54]. Prostate cancer survivors are at risk of a wide range of symptoms as a result of their cancer and its treatment [6, 55] and, while it was not our intention to explore which symptoms co-occur among prostate cancer survivors, research to investigate this would be valuable.
Conclusions
This study indicates that one in every 13 prostate cancer survivors experiences the pain-fatigue-depression symptom cluster at levels considered clinically important. Physical after-effects of prostate cancer treatment are noteworthy indicators of this cluster. More attention should be paid to identification and the support of survivors who experience multiple symptoms; this may help improve HRQoL among the growing population of prostate cancer survivors.
References
Bray F, Ferlay J, Soeromataram I et al (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68(6):394–424
Wong MCS, Goggins WB, Wang HHX et al (2016) Global incidence and mortality for prostate cancer: analysis of temporal patterns and trends in 36 countries. Eur Urol 70(5):862–874
DeSantis CE, Lin CC, Mariotto AB, Siegel RL, Stein KD, Kramer JL, Alteri R, Robbins AS, Jemal A (2014) Cancer treatment and survivorship statistics. CA Cancer J Clin 64(4):252–271
Sharp L, Deady S, Gallagher P et al (2014) The magnitude and characteristics of the population of cancer survivors: using population-based estimates of cancer prevalence to inform service planning for survivorship care. BMC Cancer 14:767
Hamdy FC, Donovan J, Lane AJ et al (2016) 10-year outcomes after monitoring, surgery, or radiotherapy for localized prostate cancer. N Engl J Med 375(15):1415–1424
Bernat JK, Wittman DA, Hawley ST et al (2016) Symptom burden and information needs in prostate cancer survivors: a case for tailored long-term survivorship care. BJU Int 118(3):372–378
Harrington CB, Hansen JA, Moskowitz M, Todd BL, Feuerstein M (2010) It's not over when it's over: long-term symptoms in cancer survivors--a systematic review. Int J Psychiatry Med 40(2):163–181
van Stam MA, van der Poel HG, Bosch JLHR et al (2017) Prevalence and correlates of mental health problems in prostate cancer survivors: a case-control study comparing survivors with general population peers. Urol Oncol 35(8):531.e1–531.e7
Aktas A, Walsh D, Rybicki L et al (2010) Symptom clusters: myth or reality? Palliat Med 24(4):373–385
Kirkova J, Walsh D, Aktas A, Davis MP (2010) Cancer symptom clusters: old concept but new data. Am J Hosp Palliat Care 27(4):282–288
Jhamb M, Abdel-Kader K, Yabes J et al (2019) Comparison of fatigue, pain, and depression in patients with advanced kidney disease and cancer – symptom burden and clusters. J Pain Symptom Manag 57(3):566–575
Kelly DL, Dickinson K, Hsiao CP, Lukkahatai N, Saligan LN (2016) Biological basis for the clustering of symptoms. Semin Oncol Nurs 32(4):351–360
Miaskowski C, Barsevick A, Berger A, Casagrande R, Grady PA, Jacobsen P, Kutner J, Patrick D, Zimmerman L, Xiao C, Matocha M, Marden S (2017) Advancing symptom science through symptom cluster research: expert panel proceedings and recommendations. J Natl Cancer Inst 109(4)
Langston B, Armes J, Levy A, Tidey E, Ream E (2013) The prevalence and severity of fatigue in men with prostate cancer: a systematic review of the literature. Support Care Cancer 21(6):1761–1771
Pettersson N, Olsson C, Tucker SL et al (2013) Urethral pain among prostate cancer survivors 1 to 14 years after radiation therapy. Int J Radiat Oncol Biol Phys 85(1):e29–e37
Watts S, Leyden G, Birch B et al. (2014) Depression and anxiety in prostate cancer: a systematic review and meta-analysis of prevalence rates. BMJ open;4(3):e003901
Storey DJ, McLaren DB, Atkinson MA, Butcher I, Liggatt S, O'Dea R, Smyth JF, Sharpe M (2012) Clinically relevant fatigue in recurrence-free prostate cancer survivors. Ann Oncol 23(1):65–72
Drummond FJ, Kinnear H, Donnelly C et al (2015) Establishing a population-based patient-reported outcomes study (PROMs) using national cancer registries across two jurisdictions: the prostate cancer treatment, your experience (PiCTure) study. BMJ Open 5(4):e006851
Gavin AT, Drummond FJ, Donnelly C, O'Leary E, Sharp L, Kinnear HR (2015) Patient-reported 'ever had' and 'current' long-term physical symptoms after prostate cancer treatments. BJU Int 116(3):397–406
Drummond FJ, O'Leary E, Gavin A, Kinnear H, Sharp L (2016) Mode of prostate cancer detection is associated with the psychological wellbeing of survivors: results from the PiCTure study. Support Care Cancer 24(5):2297–2307
Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, Filiberti A, Flechtner H, Fleishman SB, de Haes JC (1993) The European Organisation for Research and Treatment of Cancer QLQ-C30: a quality of life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 85:365–376
Lovibond SH, Lovibond PF (1995) Manual for the depression anxiety stress scales, 2nd edn. Psychology Foundation, Sydney
Fayers P, Aaronson NK, Bjordal K, Groenvold M, Curran D, Bottomley A (2001) EORTC QLQ-C30 scoring manual, 3rd edn. European Organisation for Research and Treatment of Cancer, Brussels
Giesinger JM, Kuijpers W, Young T et al (2016) Thresholds for clinical importance for four key domains of the EORTC QLQ-C30: physical functioning, emotional functioning, fatigue and pain. Health Qual Life Outcomes 14:87
Fleishman SB (2004) Treatment of symptom clusters: pain, depression, and fatigue. J Natl Cancer Inst Monogr (32):119–123
Dong ST, Butow PN, Costa DSJ, Lovell MR, Agar M (2014) Symptom clusters in patients with advanced cancer: a systematic review of observational studies. J Pain Symptom Manag 48(3):411–450
Reyes-Gibby CC, Swartz MD, Yu X, Wu X, Yennurajalingam S, Anderson KO, Spitz MR, Shete S (2013) Symptom clusters of pain, depressed mood, and fatigue in lung cancer: assessing the role of cytokine genes. Support Care Cancer 21(11):3117–3125
Laird BJ, Scott AC, Colvin LA, McKeon A, Murray GD, Fearon KC, Fallon MT (2011) Pain, depression, and fatigue as a symptom cluster in advanced cancer. J Pain Symptom Manag 42(1):1–11
Hauser K, Rybicki L, Walsh D (2006) Do pain, depression and fatigue cluster in advanced cancer? J Clin Oncol 24(18):8522
Global Cancer Observatory. 27-Prostate cancer factsheet. Available at URL https://gco.iarc.fr/today/data/factsheets/cancers/27-Prostate-fact-sheet.pdf. Accessed 1st March 2019
Wong LM, Neal DE. (2010) Where are we now with prostate cancer diagnosis? Trends Urol Men’s Health 18–20
Collin SM, Metcalfe C, Donovan J, Lane JA, Davis M, Neal D, Hamdy F, Martin RM (2008) Associations of lower urinary tract symptoms with prostate-specific antigen levels, and screen-detected localized and advanced prostate cancer: a case-control study nested within the UK population-based ProtecT (prostate testing for cancer and treatment) study. BJU Int 102(10):1400–1406
Xiao H, Tan F, Goovaerts P, Adunlin G, Ali AA, Gwede CK, Huang Y (2016) Impact of comorbidities on prostate cancer stage at diagnosis in Florida. Am J Mens Health 10(4):285–295
National Cancer Registry Ireland. Cancer Trends 30. Prostate Cancer. NCRI, Cork, 2016. Available at URL: https://www.ncri.ie/sites/ncri/files/pubs/prostateTrends2016.pdf. Accessed 1st December 2019
Gavin AT, Donnelly D, Donnelly C, Drummond FJ, Morgan E, Gormley GJ, Sharp L (2016) Effect of investigation intensity and treatment differences on prostate cancer survivors physical symptoms, psychological well-being and health-related quality of life: a two country cross-sectional study. BMJ Open 6(12):e012952
Wassersug RJ, Oliffe JL (2009) The social context for psychological distress from iatrogenic gynecomastia with suggestions for its management. J Sex Med 6(4):989–1000
Grunfeld EA, Halliday A, Martin P, Drudge-Coates L (2012) Andropause syndrome in men treated for metastatic prostate cancer: a qualitative study of the impact of symptoms. Cancer Nurs 35(1):63–69
Horrocks S, Somerset M, Stoddart H, Peters TJ (2004) What prevents older people from seeking treatment for urinary incontinence? A qualitative exploration of barriers to the use of community continence services. Fam Pract 21(6):689–696
Sciarra A, Gentilucci A, Salciccia S et al (2018) Psychological and functional effect of different primary treatments for prostate cancer: a comparative prospective analysis. Urol Oncol 36(7):340.e7–340.e21
Mols F, Korfage IJ, Vingerhoets AJ et al (2009) Bowel, urinary, and sexual problems among long-term prostate cancer survivors: a population-based study. Int J Radiat Oncol Biol Phys 73(1):30–38
Maguire R, Drummond FJ, Hanly P, Gavin A, Sharp L (2019) Problems sleeping with prostate cancer: exploring possible risk factors for sleep disturbance in a population-based sample of survivors. Support Care Cancer
Medysky ME, Temesi J, Culos-Reed SN, Millet GY (2017) Exercise, sleep and cancer-related fatigue: are they related? Neurophysiol Clin 47(2):111–122
World Health Organization (1986) Cancer pain relief. World Health Organization, Geneva
Nakano J, Hashizume K, Fukushima T, Ueno K, Matsuura E, Ikio Y, Ishii S, Morishita S, Tanaka K, Kusuba Y (2018) Effects of aerobic and resistance exercises on physical symptoms in cancer patients: a meta-analysis. Integr Cancer Ther 17(4):1048–1058
Massie MJ, Holland JC (1989) Overview of normal reactions and prevalence of psychiatric disorders. In: Holland JC, Rowland JH (eds) Handbook of psycho-oncology: psychological care of the patient with cancer. Oxford University Press, New York, pp 273–282
Fong DY, Ho JW, Hui BP et al (2012) Physical activity for cancer survivors: meta-analysis of randomised controlled trials. BMJ 344:e70
Larkin D, Lopez V, Aromataris E (2014) Managing cancer-related fatigue in men with prostate cancer: a systematic review of non-pharmacological interventions. Int J Nurs Pract 20(5):549–560
Bower JE (2014) Cancer-related fatigue—mechanisms, risk factors, and treatments. Nat Rev Clin Oncol 11(10):597–609
Drummond FJ, Gavin AT, Sharp L (2017) Supportive medications and interventions received by prostate cancer survivors: results from the PiCTure study. J Comm Support Oncol 15(6):e309
Dong ST, Butow PN, Agar M, Lovell MR, Tong A (2016) Clinicians’ perspectives on managing symptom clusters in advanced cancer: a semistructured interview study. J Pain Symptom Manag 51(4):706–71751
Kecke S, Ernst J, Einenkel J, Singer S, Hinz A (2017) Psychometric properties of the fatigue questionnaire EORTC QLQ-FA12 in a sample of female cancer patients. J Pain Symptom Manag 54(6):922–928
Zucca AC, Boyes AW, Linden W, Girgis A (2012) All’s well that ends well? Quality of life and physical symptom clusters in long-term cancer survivors across cancer types. J Pain Symptom Manag 43(4):720–731
Nho JH, Kim SR, Park MH, Kweon SS (2018) Symptom clusters and quality of life in breast cancer survivors after cancer treatment in a tertiary hospital in Korea. Eur J Cancer Care (Engl) 27(6):e12919
Kim M, Kim K, Lim C, Kim JS (2018) Symptom clusters and quality of life according to the survivorship stage in ovarian cancer survivors. West J Nurs Res 40(9):1278–1300
Watson E, Shinkins B, Frith E, Neal D, Hamdy F, Walter F, Weller D, Wilkinson C, Faithfull S, Wolstenholme J, Sooriakumaran P, Kastner C, Campbell C, Neal R, Butcher H, Matthews M, Perera R, Rose P (2016) Symptoms, unmet needs, psychological well-being and health status in survivors of prostate cancer: implications for redesigning follow-up. BJU Int 117(6B):E10–E19
Acknowledgements
The authors thank the healthcare professionals who facilitated the study; members of Men Against Cancer (MAC) and local cancer support groups who assisted with survey pre-testing; Dr. Heather Kinnear for managing the survey in NI; Joanne Clooney, Claire O’Callaghan and Audrey Craven-Lynn for survey administration and clerical support; Jenalee Kennedy, Patricia McDowell and Jonathan Mitchell for data entry; Sandra Deady and Colin Fox for providing cancer registration data; registration, data and IT staff in the registries; and, most importantly, the men who participated.
Funding
Data collection was funded by grants from the Health Research Board (HRA_HSR/2010/17), Prostate Cancer UK (NI09-03 & NI-PG13-001) and Northern Ireland R&D, with additional support from the National Cancer Control Programme in RoI. The funders had no role in the study design; collection, interpretation or analysis of data; writing the report; or the decision to submit for publication. The data analysis was undertaken as part of a degree in Biomedical Science at Newcastle University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None of the authors have any conflicts of interest to declare. The authors (via the corresponding author, LS) have full control of all primary data and agree to allow the journal to review this data if requested.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Baden, M., Lu, L., Drummond, F.J. et al. Pain, fatigue and depression symptom cluster in survivors of prostate cancer. Support Care Cancer 28, 4813–4824 (2020). https://doi.org/10.1007/s00520-019-05268-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-019-05268-0