Abstract
Objectives
Pharmacovigilance education is essential since adverse drug reactions (ADRs) are a serious health problem and contribute to unnecessary patient burden and hospital admissions. Healthcare professionals have little awareness of pharmacovigilance and ADR reporting, and only few educational interventions had durable effects on this awareness. Our future healthcare providers should therefore acquire an adequate set of pharmacovigilance competencies to rationally prescribe, distribute, and monitor drugs. We investigated the pharmacovigilance and ADR-reporting competencies of healthcare students to identify educational interventions that are effective in promoting pharmacovigilance.
Methods
The PubMed, EMBASE, Cochrane, CINAHL, PsycINFO, and ERIC databases were searched using the terms “pharmacovigilance,” “students,” and “education.”.
Results
Twenty-five cross-sectional and 14 intervention studies describing mostly medical and pharmacy students were included. Intentions and attitudes on ADR reporting were overall positive, although most students felt inadequately prepared, missed the training on this topic, and lacked basic knowledge. Although nearly all students observed ADRs during clinical rounds, only a few had actually been involved in reporting an ADR. Educational interventions were predominately lectures, sometimes accompanied by small interactive working groups. Most interventions resulted in a direct increase in knowledge with an unknown long-term effect. Real-life learning initiatives have shown that healthcare students are capable of contributing to patient care while increasing their ADR-reporting skills and knowledge.
Conclusions
There is an urgent need to improve and innovate current pharmacovigilance education for undergraduate healthcare students. By offering real-life pharmacovigilance training, students will increase their knowledge and awareness but can also assist current healthcare professionals to meet their pharmacovigilance obligations.
Similar content being viewed by others
Introduction
Most healthcare students enter clinical practice immediately after graduation and are required to prescribe, distribute, administer, and/or monitor drugs on a daily basis. In order to perform these responsibilities effectively and to ensure the safe use of medications, healthcare students (especially in medicine, pharmacy, dentistry, and nursing curricula) should acquire a minimum set of pharmacovigilance competencies before they graduate and start clinical practice [1, 2]. Foreseeing, recognizing, managing, and reporting adverse drug reactions (ADRs) are an important part of rational and safe prescribing and are integrated into multiple steps of the WHO-six-step Guide to Good Prescribing [3]. It is a professional responsibility of all healthcare professionals. Despite this, healthcare curricula often teach little on pharmacovigilance and ADR reporting, with a median of 4–5.5 contact hours [4]. Numerous studies have expressed concern about the lack of healthcare professional competencies in pharmacovigilance [4,5,6].
This lack of undergraduate education and training in pharmacovigilance is consistent with the low level of knowledge, skills, and actions seen not only in physicians but also in practicing pharmacists, dentists, and nurses [7,8,9]. Unfamiliarity with pharmacovigilance, a low level of ADR-reporting skills, a lack of knowledge combined with negative attitudes like ignorance, fear legal liability, and lack of importance are thought to be related to the current inadequate response to many ADRs [10,11,12,13]. Several interventions (implementing protocols, educational workshops, or repeated emailing or telephone calls) have been implemented in an attempt to improve the competence of healthcare professionals [14,15,16,17], but these interventions are costly or fail to produce clinically relevant and long-term effects [8].
Despite the urgency of this problem, each year millions of medication users experience ADRs ranging from minor discomfort to hospital admission, permanent disability, or even death [18]. ADRs are responsible for 3.0–6.5% of all hospital admissions, 0.15% of all deaths, and could have been prevented in 47–72% of cases by good pharmacological and pharmacovigilance skills and knowledge [19,20,21,22].
Pharmacovigilance centers have an important role in the dissemination of current pharmacovigilance knowledge. Their data are mainly based on post-marketing reporting, which is essential for identifying previously undetected, uncommon, or serious ADRs. In most countries, pharmacovigilance center causality assessments of ADRs rely on a mixture of spontaneous reporting by healthcare professionals (physicians, pharmacists, nurses, and dentists) and patients. Since healthcare professionals have a different focus in ADR reporting, it is important to involve all parties [23,24,25,26,27]. With population aging, the increased use of prescription drugs and polypharmacy will probably lead to a drastic rise in the number of ADRs [28]. This together with ADR underreporting [29, 30] and the lack of awareness and understanding of ADRs could lead to an even greater burden on patients and healthcare systems in the near future.
By studying the pharmacovigilance and ADR-reporting competencies of healthcare students, we aim to identify effective educational interventions that promote pharmacovigilance early in their education and career. The primary objectives of this review were therefore to analyze the following: (1) what is known about the pharmacovigilance competencies of healthcare students and (2) which educational interventions are effective in pharmacovigilance education.
Methods
General methodology
We searched the literature to analyze the current level of competencies and the effects of different undergraduate pharmacovigilance interventions, using the Kirkpatrick model of hierarchy of evaluation, as modified by Freeth [31]. Given the diverse outcome measures, no meta-analysis was performed.
Search strategy
With assistance of a medical information specialist (R.O.), the MEDLINE (PubMed), EMBASE, PsycINFO, Cochrane, CINAHL, and ERIC databases were searched for articles on pharmacovigilance education. MEDLINE was used as the standard medical research database. The Embase, PsycINFO, Cochrane, and CINAHL databases were used for articles published in biomedical and nursing databases. The ERIC database functioned as a supplementary detector for educational articles. All databases were searched until February 1, 2017, with database-specific queries [S4] without additional filters. All queries used “pharmacovigilance,” “students,” and “education” or commonly used abbreviations of similar terms (e.g., adverse drug reporting systems, undergraduate, and teaching, respectively). Articles were retrieved from the local university library or requested from the original authors, institution, or publisher. The references of relevant articles were screened using the snowball method [32].
Study selection
First, two authors (MR and BP) independently screened all articles for eligibility based on their titles and preset inclusion and exclusion criteria [Supplement Table 1]. If there was any discrepancy about the content of the article, the abstract (if available) and/or full article was screened. Disagreements were resolved by mutual consensus. All eligible abstracts and articles were assessed in a similar way. Articles were included if they analyzed pharmacovigilance competencies in undergraduate healthcare students. Articles were not limited to the study setting, country of origin, or publication date. Exclusion criteria were as follows: (1) outcome measure not related to the pharmacovigilance competencies; (2) evaluation of a specialty-specific ADR; (3) undergraduate healthcare students were not studied (e.g., healthcare professionals or patients); (4) language other than English or Dutch; (5) studying medical or dietary supplements, herbal products, or alternative medicines; and (6) non-original research studies (e.g., reviews, editorials, letters to the editor, and conference abstracts).
Data extraction
Data were extracted by two authors [MR and BP] using a modified coding sheet, based on the Best Evidence Medical Education (BEME) Collaboration coding sheet [33, 34]. This modified coding sheet included the study design and aim, instruments used, characteristics of the educational intervention, students’ educational level and performance, overall conclusion, and recommendations. The Kirkpatrick model of hierarchy of evaluation, modified by Freeth [31], was added to evaluate the outcome level.
Quality assessment
Study quality was assessed using the Medical Education Research Study Quality Instrument (MERSQI) [35]. This instrument was developed to assess educational studies and consists of six domains: study design, sampling, type of data, validity of the evaluation instrument, data analysis, and outcomes. Scores range from 5 to 18 points. Although there is no defined cutoff for high- or low-quality study methods, a previous study considered scores of 5–8.5 to reflect a low-quality study method, 9.0–13.0 to reflect a moderate-quality study method, and 13.5–18 as a high-quality study method [36].
Data analysis
Data were analyzed using SPSS Statistics 22 (Chicago, IL). Descriptive statistics were used to report total mean MERSQI scores, proportion of articles with a different country of origin, type of healthcare student, and study design. The MERSQI scores of the main groups of student outcomes were compared using a one-way ANOVA with an alpha of < 0.05.
Given the differences in study design and outcome measures, only a quantitative analysis was possible. Student motives for reporting ADRs were described using descriptive statistics. Student opinions on educational aspects were recoded into three groups (No: ≤ 33% of students (fully) agreed, Neutral: ≥ 34 ≤ 66% of students (fully) agreed, Yes: ≥ 67% of students (fully) agreed.
Results
Search results
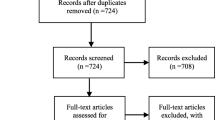
The initial search identified 2468 unique articles. Figure 1 shows the flowchart of the search, selection, and review process. Thirty-three articles were eligible for inclusion. The 727 references of the 33 eligible articles were snowball searched, which yielded 6 new articles. In total, 14 intervention and 25 cross-sectional articles were included in our analysis.
Acquired pharmacovigilance competencies
As shown in Table 1, there is no uniform pharmacovigilance evaluation method. Most articles studied ADR reporting and pharmacovigilance knowledge (Kirkpatrick level 2b) in undergraduate medical and pharmacy students. Two studies [41, 42] used identical research and outcome measures and have been compared separately [61].
Twenty-two articles analyzed student opinions, intentions, and attitudes to ADR reporting and pharmacovigilance. Between 53 and 100% of students agreed that ADR reporting was a professional responsibility [42, 46,47,48], and most articles concluded that pharmacists were the most important healthcare professionals for this [37, 43, 47, 51]. However, all students agreed that all healthcare professionals should be aware of ADRs and ADR reporting [49, 58]. Students had favorable intentions about reporting ADRs (5.9 ± 1.5 to 6.17 ± 0.95; 1–7 min/max) and would try to report (6.0 ± 1.3 to 6.10 ± 1.0; 1–7 min/max) serious ADRs during their internships/clerkships [6, 40]. A large proportion (73.5–75.6%) of students agreed that ADR reporting should be compulsory for pharmacists [39, 43, 53].
Almost all articles analyzed students’ knowledge to some degree, although skills were not analyzed in the cross-sectional studies. Overall knowledge was poor, since only half (37.5–80%) of the students were familiar with the term “adverse drug reactions” [37, 41, 42, 45,46,47, 53, 57], “pharmacovigilance” (18–66%) [41, 42, 45,46,47, 53, 57], and the clinical relevance of pharmacovigilance (19–63%) [41, 42, 46, 57]. In contrast, students’ knowledge of the ADR classification of Rawlins [62], a more challenging topic, was known in these two studies [47, 53].
Fourteen articles analyzed what students did in practice in terms of pharmacovigilance and ADR reporting. Although many students (median 63%, IQR 63–87%) had encountered an ADR during their clinical training [41, 44, 45, 48, 58], only a few (median 10%, IQR 13%) had previously been involved in reporting an ADR [39,40,41,42, 45, 58, 63]. Most students did not know where to report an ADR (median 57%, IQR 47–91%) [37, 39, 40, 46,47,48, 56], which method they should be used to report an ADR (median 72% IQR 62–86%) [38, 41, 42, 45, 46], or how to get access to the ADR report form (median 84%, IQR 61–92%) [41,42,43, 48, 54].
Sixteen studies analyzed students’ opinions of their perceived level of training in pharmacovigilance and ADR reporting (Supplement Fig. 1). One study of pharmacy students concluded that students felt sufficiently trained [37]. Conversely, six studies of pharmacy and medical students showed that students felt inadequately qualified to report ADRs or to perform pharmacovigilance [41,42,43,44, 48, 51]. Additionally, three (27%) studies reported that fourth- and fifth-year medical and pharmacy students also felt to have inadequate knowledge to report ADRs [42, 49, 51]. Healthcare students in almost all (15 studies) studies felt that ADR reporting and pharmacovigilance should be included in pharmacy and medical curricula [38, 40,41,42,43, 45, 47, 48, 53, 57, 59, 64,65,66]. Two studies reported that dentistry [46] and nursing [56] students felt neither positive nor negative about including ADR reporting in their curriculum.
Seven studies individually analyzed student reasons for reporting or not reporting ADRs to the competent authority [6, 37, 39, 40, 43, 47, 67] (Supplement Table 3). A lack of encouragement (n = 3), lack of information provided by patients (n = 2), and a lack of knowledge on how to report (n = 2) were the reasons most often given for not reporting ADRs. Educating others (n = 3), improving patient safety (n = 3), and contributing to the safe use of medicines (n = 3) were the reasons most often given for reporting ADRs.
What factors influence pharmacovigilance competencies?
Two comparative studies investigated differences in attitude and knowledge to pharmacovigilance and ADR reporting between medical and pharmacy students [47, 57]. Sivadasan et al. [45, 57] showed that more medical students than pharmacy students considered ADR reporting to be essential (80.5 vs 75.8%) and considered it their professional responsibility (69 vs 51.6%) [45]. Conversely, Umair Khan et al. showed that significantly more pharmacy students than medical students considered ADR reporting as important as managing patients (79.1 vs 43.5%) [47]. Both studies concluded that final-year pharmacy students had superior pharmacovigilance knowledge compared with medical students: 5.61 ± 1.78 vs 3.23 ± 1.60, 0–10 min/max and 8.4 ± 0.2 vs 3.17 ± 0.06; 0–15 min/max, respectively [47, 57].
Additional comparisons between gender, race/ancestry, pharmacology curricula, previous pharmacovigilance or ADR-reporting training, previous ADR-reporting experience, and level of professional year were analyzed to identify factors associated with a higher level of pharmacovigilance competence. Race/ancestry did not influence pharmacovigilance knowledge, although male students knew more about post-marketing surveillance and female students knew more about causality assessments [38]. Overall, PharmD (Master of Pharmacy) students had more positive attitudes and higher knowledge scores than BPharm (Bachelor of Pharmacy) students [37, 43], probably because the former had trained for longer. A positive correlation was found between student knowledge and their skills in ADR reporting (r 0.485, p < 0.001) [50]. Previous training in ADR reporting or reporting experience was associated with significantly higher student knowledge scores [40, 47, 53]. In line with these observations, academically older students had more knowledge, were more aware of ADRs during their internships, and had reported more ADRs.
Which pharmacovigilance interventions are effective?
There is no uniform pharmacovigilance educational intervention (Table 2). Interventions have ranged from short 15-min power point lectures and multiple training workshops to more innovative clinical experiences in ADR reporting or assessment. No replicated intervention studies have been published to our knowledge.
Four articles evaluated student satisfaction regarding pharmacovigilance education [65, 67, 75, 77]. Students found clinical experience more educational than lectures and/or solving fictional casuistry [67]. Students also stressed that pharmacovigilance training should be repeated during the internships [77]. Six articles examined students’ intentions and attitudes toward ADR reporting after a pharmacovigilance intervention [65,66,67, 73, 74, 77]. However, since none of the studies included a baseline assessment and substantial differences were not observed between cross-sectional and intervention studies, it was not possible to draw conclusions.
Two studies by Durrieu et al. focused on students’ perception of the risk of ADRs [73, 74]. They concluded that after a pharmacology course, students were more aware of potentially serious ADRs. A follow-up study showed that perception of the risks of ADRs was more clinically realistic after clinical training, i.e., students were more aware of potentially serious ADRs associated with anticoagulants and non-steroidal anti-inflammatory drugs (NSAIDs) and less conservative about hypercholesterolemia drugs.
Five studies showed a significant increase in pharmacovigilance and ADR-reporting knowledge scores directly after the intervention was completed [66,67,68,69, 71]. Since most studies asked different pharmacovigilance questions or used grouped outcome scores [68, 69, 71], it was not possible to state that one intervention was superior to another. Studies with a longer follow-up time (1–12 months) reported contrasting outcomes. Two studies showed a significant increase in pharmacovigilance knowledge and ADR-reporting skills after 1 and 6 months [71, 77]. However, Arici et al. reported a significant increase in pharmacovigilance knowledge directly after an intervention, but this had faded by 12 months [68].
Three studies analyzed pharmacovigilance or ADR-reporting skills [60, 67, 77]. Schutte et al. showed that medical students were significantly more aware of the importance of ADR reporting after assessing a real ADR report themselves [67]. Tripathi et al. and Rosebraugh et al. analyzed the impact of an intervention on the quality of completing a fictional ADR report in undergraduate medical students [60, 77]. Both showed that a 15-min lecture significantly increased the quality of an ADR report.
Four articles analyzed pharmacovigilance competences in a real-life clinical setting [67, 70, 72, 76], three of which involved pharmacy students [70, 72, 76]. Findings suggested that pharmacy students could play an important part in regular pharmacovigilance healthcare. Christensen et al. and Sullivan and Spooner found a significant increase in the number of ADRs reported in a hospital setting [72, 76], and Armando et al. found that second-year pharmacy students were equally capable of recognizing ADRs in a community pharmacy setting as pharmacists [70]. Schutte et al. showed that medical students were also capable of assessing real ADR reports [67].
Discussion
We found that while healthcare students have favorable intentions and positive attitudes toward ADR reporting, most lack the basic skills and knowledge to do so. Overall, academically older students and students with prior pharmacovigilance experience were more competent in recognizing and reporting ADRs. Pharmacy students had slightly more knowledge of pharmacovigilance and ADR reporting than other healthcare students. Students agreed that pharmacists are the most important healthcare professional with regard to pharmacovigilance, although all students felt responsible for pharmacovigilance. Students perceived their knowledge to be moderate at best, felt they did not receive sufficient training, and stated that pharmacovigilance and ADR reporting should be included in their curriculum. It is not surprising that while relatively many students had seen an ADR (63%), few had reported one (10%). This is consistent with previous studies [78] and the current low rate of ADR reporting (medial reporting rate of 6%) [29] among qualified health professionals.
Despite this lack of competence in pharmacovigilance and ADR reporting, we identified 14 studies that reported beneficial effects of an intervention. Students valued real and legitimate pharmacovigilance tasks, such as diagnosing, reporting, or assessing ADR reports, more than outdated educational interventions or fictional casuistry. This type of clinical training also leads to a more clinically realistic perception of the risk of ADRs. Although educational pharmacovigilance interventions ultimately aim at a clinically relevant and long-term increase in medication safety, no study has looked at this highest hierarchical level. Most outdated interventions only provide a short-term increase in knowledge, few show clinically relevant results, and none has shown durable clinical outcomes. Repeated clinical training which boosts intrinsic motivation and improves learning outcomes [79, 80] should be applied to pharmacovigilance training. Additionally, the interventions that focused on real and legitimate clinical tasks, such as diagnosing and reporting ADRs and assessing ADR reports, also had a positive effect on the healthcare system. Multiple studies have shown the clinical value of student participation in pharmacovigilance tasks.
Although our findings are worrying, the outcome should be interpreted with some caution given the heterogeneity and methodological weaknesses of the included studies. All intervention studies were single institution, had variable intervention designs, used different assessment methods of no clear relevance, and were ultimately of moderate study quality (mean MERSQI score 11.1). Since this is the first systematically performed review to investigate the current pharmacovigilance competencies of all types of healthcare students, we cannot compare our findings with those of other studies. A similar review, focusing on only a few competencies in medical students, reported similar outcomes [5].
This review had a number of limitations. Articles may have been missed, although we attempted to reduce the likelihood of this by searching six databases and using a snowball strategy. Overall, the studies were only of moderate quality, with low response rates, and small intervention groups, many of which had not been retested. Despite these weaknesses and the possibility that student capabilities were overestimated, because of publication bias, most competencies are still far from satisfactory. Moreover, the heterogeneity of assessment instruments used, outcome measures, and interventions, in combination with the combined competency scores in some studies, made a full comparison or meta-analysis impossible. However, this heterogeneity could mask some interesting features, since only few frequently reported variables were studied in detail. Lastly, the difference in location of cross-sectional studies (66% in Asia) and intervention studies (24% in Asia) may have skewed the analyses.
Conclusion
This review highlights the urgent need to improve and modernize current pharmacovigilance education for undergraduate healthcare students. However, the best way to provide this education still needs to be established, but the content of pharmacovigilance education should at least be as real as possible. We suggest it is given real life context, i.e., with clinical relevance as early responsibility for the student (under supervision). It should be integrated into different healthcare curricula (medicine, pharmacy, dentistry, and nursing) and repeated throughout academic training, starting as early as possible, in the Bachelor phase. By offering real clinical pharmacovigilance training, students can not only increase their knowledge, awareness, and skills, but can also assist current healthcare professionals meet their clinical pharmacovigilance obligations. Future research should therefore focus on valid and reliable methods for assessing pharmacovigilance competencies in clinical practice. To successfully develop and initiate pharmacovigilance educational programs, further work is needed to evaluate educational interventions on Kirkpatrick’s highest hierarchical levels, preferably in an inter-professional setting, with a multicenter design and a long follow-up. Internships or student-run clinics may be useful since they offer students early pharmacovigilance experiences with real responsibilities for patient care, with the advantage of assisting current healthcare professionals, limiting the level of underreporting, and ultimately preventing ADRs and increasing patient safety.
References
Brinkman DJTJ, Mokkink LB, Christiaens T, Likic R, Maciulaitis R, Costa J, Sanz EJ, Maxwell SR, Richir MC, van Agtmael MA, Education Working Group of the European Association for Clinical Pharmacology and Therapeutics (2017) Key learning outcomes for clinical pharmacology and therapeutics education in Europe: a modified Delphi study. Clin Pharmacol Ther
Maxwell SR, Cascorbi I, Orme M, Webb DJ, Joint BPS/EACPT Working Group on Safe Presribing (2007) Educating European (junior) doctors for safe prescribing. Basic Clin Pharmacol Toxicol 101:395–400
de Vries TPGM, Henning RH, Hogerzeil HV, Fresle DA (1994) Guide to good prescribing—a practical manual. WHO, Geneva
Jenny Hartman LH, van Puijenbroek E (2017) A global view of undergraduate education in pharmacovigilance. Eur J Clin Pharmacol 73:891–899
Abdullahi Rabiu Abubakar MH (2016) Pharmacovigilance practice: the current challenges and the gaps in the medical students’ curriculum. J App Pharm Sci 6(05):210–2015
Gavaza P, Bui B (2012) Pharmacy students' attitudes toward reporting serious adverse drug events. Am J Pharm Educ 76(10):194
De Angelis A, Colaceci S, Giusti A, Vellone E, Alvaro R (2015) Factors that condition the spontaneous reporting of adverse drug reactions among nurses: an integrative review. J Nurs Manag 23(4):1–13p
Pagotto C, Varallo F, Mastroianni P (2013) Impact of educational interventions on adverse drug events reporting. Int J Technol Assess Health Care 29(4):410–417
Rutter P, Brown D, Howard J, Randall C (2014) Pharmacists in pharmacovigilance: can increased diagnostic opportunity in community settings translate to better vigilance? Drug Saf 37(7):465–469
Gonzalez-Gonzalez C, Lopez-Gonzalez E, Herdeiro MT, Figueiras A (2013) Strategies to improve adverse drug reaction reporting: a critical and systematic review. Drug Saf 36(5):317–328
Lopez-Gonzalez E, Herdeiro MT, Figueiras A (2009) Determinants of under-reporting of adverse drug reactions: a systematic review. Drug Saf 32(1):19–31
WH I (1996) Attitudes to adverse drug reaction reporting. Br J Clin Pharmacol 41(5):434–435
Alessia De Angelis SC, Giusti A, Vellone E, Alvaro R (2015) Factors that condition the spontaneous reporting of adverse drug reactions among nurses: an integrative review. J Nurs Manag 24(2)
Herdeiro MT, Ribeiro-Vaz I, Ferreira M, Polnia J, Falco A, Figueiras A (2012) Workshop-and telephone-based interventions to improve adverse drug reaction reporting: a cluster-randomized trial in Portugal. Drug Saf 35(8):655–665
Johansson-Pajala RM, Martin L, Fastbom J, Blomgren KJ (2015) Nurses’ self-reported medication competence in relation to their pharmacovigilant activities in clinical practice. J Eval Clin Pract 21(1):145–152
Ribeiro-Vaz I, Santos CC, Cruz-Correia R (2016) Promoting adverse drug reaction reporting: comparison of different approaches. Rev Saude Publica 50:14
Ribeiro-Vaz IH, T M, Polonia J, Figueiras A (2011) Strategies to increase the sensitivity of pharmacovigilance in Portugal. Rev Saude Publica 45(1):129–135
Lazarou J, Pomeranz BH, Corey PN (1998) Incidence of adverse drug reactions in hospitalized patients: a meta-analysis of prospective studies. JAMA 279(15):1200–1205
Krähenbühl-Melcher A, Schlienger R, Lampert M, Haschke M, Drewe J, Krähenbühl S (2007) Drug-related problems in hospitals: a review of the recent literature. Drug Saf 30(5):379–407
Pirmohamed M, James S, Meakin S, Green C, Scott AK, Walley TJ, Farrar K, Park BK, Breckenridge AM (2004) Adverse drug reactions as cause of admission to hospital: prospective analysis of 18 820 patients. BMJ 329(7456):15–19
Gyllensten HRC, Jonsson AK, Petzold M, Carlsten A, Andersson Sundell K (2013) Cost of illness of patient-reported adverse drug events: a population-based cross-sectional survey. BMJ Open 3(6):e002574
Sultana J, Cutroneo P, Trifiro G (2013) Clinical and economic burden of adverse drug reactions. J Pharmacol Pharmacother 4(Suppl 1):S73–S77
van Grootheest K (2003) The dawn of pharmacovigilance: an historical perspective. Int J Pharmaceut Med 17:195. https://doi.org/10.2165/00124363-200317050-00006
Hall M, McCormack P, Arthurs N, Feely J (1995) The spontaneous reporting of adverse drug reactions by nurses. Br J Clin Pharmacol 40(2):173–175
Bigi CBG (2017) The key role of clinical and community health nurses in pharmacovigilance. Eur J Clin Pharmacol 73:1379–1387
Carnelio S, Khan SA, Rodrigues G (2011) Pharmacovigilance in clinical dentistry: overlooked or axiomatic? Gen Dent 59(1):24–28 quiz 9–30, 80
Schutte T, van Eekeren R, Richir M, van Staveren J, van Puijenbroek E, Tichelaar J, van Agtmael M (2017) The adverse drug reaction reporting assignment for specialist oncology nurses: a preliminary evaluation of quality, relevance and educational value in a prospective cohort study. Naunyn Schmiedeberg's Arch Pharmacol
Atkin PASG (1995) Medication-related adverse reactions and the elderly: a literature review. Adverse Drug React Toxicol Rev 14:175–191
Hazell L, Shakir SA (2006) Under-reporting of adverse drug reactions: a systematic review. Drug Saf 29(5):385–396
Backstrom M, Mjorndal T, Dahlqvist R (2004) Under-reporting of serious adverse drug reactions in Sweden. Pharmacoepidemiol Drug Saf 13(7):483–487
Freeth D, Hammick M, Koppel I, Reeves S, Barr H (2002) A critical review of evaluations of interprofessional education. Higher Education Academy Learning and Teaching Support Network. Available at https://www.caipe.org/news/freeth-d-hammick-m-koppel-i-reeves-s-barr-h-al-2002-a-critical-review-of-evaluations-of-interprofessional-education-hea-health-sciences-and-practice-occasional-paper-2
Wohlin C. Guidelines for snowballing in systematic literature studies and a replication in software engineering. [Available from: https://dl.acm.org/citation.cfm?id=2601268
Hammick MDT, Steinert Y (2010) Conducting a best evidence systematic review. Part 1: from idea to data coding. BEME Guide No 13. Med Teach 32:3–15
Steinert YMK, Centeno A, Dolmans D, Spencer J, Gelula M et al (2006) A systematic review of faculty development initiatives designed to improve teaching effectiveness in medical education: BEME Guide No. 8. Med Teach 28:497–526
Reed DACD, Beckman TJ, Levine RB, Kern DE, Wright SM (2007) Association between funding and quality of published medical education research. JAMA 298:1002–1009
Bogetz JFRC, Bereknyei S, Mendoza FS, Sanders LM, Braddock CH 3rd (2015) Training health care professionals for 21st-century practice: a systematic review of educational interventions on chronic care. Acad Med 90(11):1561–1572
Ahmad A, Khan MU, Moorthy J, Kumar BD, Kumar GS, Patel I (2016) Comparison of knowledge, attitudes and perceived barriers towards adverse drug reactions reporting between Bachelor of Pharmacy and Doctor of Pharmacy students in Southern India. J Pharm Health Serv Res 7(1):63–69
Rajiah K, Maharajan MK, Nair S (2015) Pharmacy students’ knowledge and perceptions about adverse drug reactions reporting and pharmacovigilance. Saudi Pharm J. https://doi.org/10.1016/j.jsps.2015.03.021
Saurabh MK, Karnani RK (2016) An evaluation of knowledge, attitude and perception about adverse drug reactions and pharmacovigilance among intern doctors at a teaching hospital of Rajasthan. National Journal of Physiology, Pharmacy and Pharmacology 6(2):111–115
Schutte T, Tichelaar J, Reumerman MO, van Eekeren R, Rissmann R, Kramers C, Richir MC, van Puijenbroek EP, van Agtmael MA (2017) Pharmacovigilance skills, knowledge and attitudes in our future doctors—a nationwide study in the Netherlands. Basic Clin Pharmacol Toxicol 120(5):475–481. https://doi.org/10.1111/bcpt.12712
Abubakar A, Chedi B, Mohammed K, Haque M (2015) Perception of Nigerian medical students on adverse drug reaction reporting. J Adv Pharm Technol Res 6(4):154–158
Abubakar AR, Simbak NB, Haque M (2015) Pharmacovigilance study: awareness among medical students of a new medical school of Malaysia. International Journal of Pharmaceutical Research 7(1):83–88
Farha RA, Alsous M, Elayeh E, Hattab D (2015) A cross-sectional study on knowledge and perceptions of pharmacovigilance among pharmacy students of selected tertiary institutions in Jordan. Trop J Pharm Res 14(10):1899–1905. https://doi.org/10.4314/tjpr.v14i10.23
Ismail SB, Rahman NIA, Anantrao NH, Dali WPEW, Umar BU, Haque M (2015) Awareness of pharmacovigilance among future house officers; a study among first batch of final year medical students of UniSZA, Malaysia. International Journal of Pharmaceutical Research. 7(2):96–101
Meher BR, Joshua N, Asha B, Mukherji D (2015) A questionnaire based study to assess knowledge, attitude and practice of pharmacovigilance among undergraduate medical students in a Tertiary Care Teaching Hospital of South India. Perspect Clin Res 6(4):217–221
Shalini S, Mohan S (2015) Knowledge and attitude towards pharmacovigilance and adverse drug reaction reporting among dental students in a Private University, Malaysia. J Young Pharm 7(2):118–125. https://doi.org/10.5530/jyp.2015.2.10
Umair Khan M, Ahmad A, Ejaz A, et al. (2015) Comparison of the knowledge, attitudes, and perception of barriers regarding adverse drug reaction reporting between pharmacy and medical students in Pakistan. J of Educ Eval Health Prof 12:28. https://doi.org/10.3352/jeehp.2015.12.28
Iffat W, Shakeel S, Naseem S, Imam S, Khan M (2014) Attitudinal survey to assess medical and dental students’ belief of ADR reporting in Pakistan. Int J Pharm Pharm Sci 6(5):279–283
Segun S, Fakeye T (2013) The concept of adverse drug reaction reporting: awareness among pharmacy students in a Nigerian university. Internet J Med Update 8:24–30
Hema NG, Bhuvana KB (2012) Sangeetha. Pharmacovigilance: the extent of awareness among the final year students, interns and postgraduates in a government teaching hospital. J Clin Diagn Res 6(7 SUPPL):1248–1253
Sharma S, Sharma J, Aggarwal T (2012) A survey on knowledge and perception of pharmacy students towards adverse drug reaction (ADR) reporting. Asian Journal of Pharmaceutical and Clinical Research 5(SUPPL. 3):129–131
Vora MB, Paliwal NP, Doshi VG, Barvaliya MJ, Tripathi CB (2012) Knowledge of adverse drug reactions and pharmacovigilance activity among the undergraduate medical students of GUJARAT. Int J Pharm Sci Res 3(5):1511–1515
Elkalmi RM, Hassali MA, Ibrahim MI, Widodo RT, Efan QM, Hadi MA (2011) Pharmacy students’ knowledge and perceptions about pharmacovigilance in Malaysian public universities. Am J Pharm Educ 75(5):96
Sears EL, Generali JA (2005) Adverse drug reaction and medication error reporting by pharmacy students. Ann Pharmacother 39(3):452–459. https://doi.org/10.1345/aph.1E369
Rehan HS, Vasudev K, Tripathi CD (2002) Adverse drug reaction monitoring: knowledge, attitude and practices of medical students and prescribers. Natl Med J India 15:24–26
Sivadasan SSM (2015) A study on the awareness and attitude towards pharmacovigilance and adverse drug reaction reporting among nursing students in a private university, Malaysia. Int J Curr Pharm Res 7:84–89
Sivadasan SYN, Chyi NW et al (2014) Knowledge and perception towards pharmacovigilance and adverse drug reaction reporting among medicine and pharmacy students. WJPPS
Etminani-Isfahani MAM, Mousavi S, Rakhshan A, Assarian M, Kuti L, Eslami K (2013) Adverse drug reaction: knowledge, attitude and practice of pharmacy students. Journal of Pharmaceutical Care
M. Limuaco O (2014) The extent of pharmacovigilance awareness among pharmacy senior students of Centro Escolar University, Manila, Philippines. Journal of Pharmacovigilance;02(01)
Rosebraugh CJ, Tsong Y, Zhou F, Chen M, Mackey AC, Flowers C et al (2003) Improving the quality of adverse drug reaction reporting by 4th-year medical students. Pharmacoepidemiol Drug Saf 12(2):97–101. https://doi.org/10.1002/pds.797
Abubakar AR, Ismail S, Rahman NI, Haque M (2015) Comparative study on drug safety surveillance between medical students of Malaysia and Nigeria. Ther Clin Risk Manag 11:1015–1025
JW RMT (1977) Pathogenesis of adverse drug reactions. In: DM D (ed) Textbook of adverse drug reactions. 10. Oxford University Press, Oxford
Sharma A, Amarnath S, Jaikumar S, Basalingappa S, Ramaswamy S, Thulasimani M (2014) Assessment of knowledge about pharmacovigilance among medical students in Puducherry. Research Journal of Pharmacy and Technology 7(4):447–449
Schutte T, Tichelaar J, Reumerman MO, Van Eekeren R, Rolfes L, Richir MC et al (2015) Learning by doing in the student-run pharmacovigilance program. Clin Ther 37((8):e79
Mohan L, Kumar A, Mishara MR, Kishore A, Nayak V (2012) Usefulness of training of pharmacovigilance for medical students—a perspective study. Int J Pharm Sci Rev Res 16(1):56–59
Lokesh Reddy VJP, Rathinavelu SK, Padmanabha M, Reddy Y (2014) Assessment of knowledge, attitude and perception of pharmacovigilance and adverse drug reaction (ADR) reporting among the pharmacy students in South India. Journal of Pharmacy and Biological Sciences
Schutte T, Tichelaar J, Reumerman MO, Van Eekeren R, Rolfes L, Richir MC et al (2017) Feasibility and educational value of a student-run pharmacovigilance programme: a prospective cohort study. Drug Saf 40(5):409–418
Arici AM, Gelal A, Demiral Y, Tuncok Y (2011) Short-term impacts of pharmacovigilance education on the pharmacovigilance knowledge level of fifth-year medical students. Drug Saf 34(10):1005
Amarnath S, Sharma A, Jaikumar S, Basalingappa S, Ramaswamy S, Thulasimani M (2014) Impact of an educational intervention on the awareness of pharmacovigilance among pharmacy and nursing students in Puducherry. Res J Pharm Biol Chem Sci 5(2):1130–1136
Armando P, Uema S, Solá N (2003) Pharmacotherapeutic monitoring in supervised student practice sessions: the application of Dáder methodology in the identification of ADR. Ars Pharmaceutica 44(2):185–192
Chandy SJ (2016) The need for a comprehensive medication safety module in medical education. Indian J Pharmacol. 48(Suppl 1):S57–S60
Christensen ST, Sondergaard B, Honore PH, Bjerrum OJ (2011) Pharmacy student driven detection of adverse drug reactions in the community pharmacy setting. Pharmacoepidemiol Drug Saf 20(4):399–404
Durrieu G, Hurault C, Bongard V, Damase-Michel C, Montastruc JL (2007) Perception of risk of adverse drug reactions by medical students: influence of a 1 year pharmacological course. Br J Clin Pharmacol 64(2):233–236
Durrieu G, Hurault C, Damase-Michel C, Montastruc JL (2010) Perception of risk of adverse drug reactions: a 3-year follow-up of a cohort of medical students. Fundam Clin Pharmacol 24(4):423–427
Naritoku DK, Faingold CL (2009) Development of a therapeutics curriculum to enhance knowledge of fourth-year medical students about clinical uses and adverse effects of drugs [corrected] [published erratum appears in TEACH LEARN MED 2009 Jul–Sep;21(3):279]. Teaching & Learning in Medicine 21(2):148–152 5p
Sullivan KM, Spooner LM (2008) Adverse-drug-reaction reporting by pharmacy students in a teaching hospital. Am J Health Syst Pharm 65(12):1177–1179
Tripathi RK, Jalgaonkar SV, Sarkate PV, Rege NN (2016) Implementation of a module to promote competency in adverse drug reaction reporting in undergraduate medical students. Indian J Pharmacol 48(Suppl 1):S69–S73
Brinkman DJTJ, Schutte T, Benemei S, Böttiger Y, Chamontin B, Christiaens T, Likic R, Maˇiulaitis R, Marandi T, Monteiro EC, Papaioannidou P, Pers YM, Pontes C, Raskovic A, Regenthal R, Sanz EJ, Tamba BI, Wilson K, Vries T, Richir MC, Agtmael MV (2017) Essential competencies in prescribing: a first European cross-sectional study among 895 final-year medical students. Clin Pharmacol Ther 101(2):281–289
Dekker RS, Schutte T, Tichelaar J, Thijs A, van Agtmael MA, de Vries TP et al (2015) A novel approach to teaching pharmacotherapeutics—feasibility of the learner-centered student-run clinic. Eur J Clin Pharmacol 71(11):1381–1387
Yardley STP, Dornan T (2012) Experiential learning: AMEE Guide No. 63. Med Teach:e102–ee15
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Originality and agreement statement
All of the authors declare that this work has not been and will not be published in whole or part in any other journals, and agree to the contents of the manuscript in its submitted form.
Additional information
Key points
• Undergraduate healthcare students have good intentions and positive attitudes on ADR reporting; however, they feel inadequately prepared and lack basic knowledge on this topic.
• Current pharmacovigilance education is predominantly focused on lectures, sometimes accompanied by small interactive working groups although the long-term effects of this type of education are still unknown.
• Real-life learning initiatives in pharmacovigilance have proven effective in increasing student knowledge and awareness and also assist current healthcare professionals to meet their pharmacovigilance obligations.
Electronic supplementary material
ESM 1
(DOCX 33 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Reumerman, M., Tichelaar, J., Piersma, B. et al. Urgent need to modernize pharmacovigilance education in healthcare curricula: review of the literature. Eur J Clin Pharmacol 74, 1235–1248 (2018). https://doi.org/10.1007/s00228-018-2500-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-018-2500-y